Arachidonic acid
 | |||
| |||
| Names | |||
|---|---|---|---|
IUPAC name (5Z,8Z,11Z,14Z)-5,8,11,14-Eicosatetraenoic acid | |||
Systematic IUPAC name (5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid[1] | |||
| Other names 5,8,11,14-all-cis-Eicosatetraenoic acid; all-cis-5,8,11,14-Eicosatetraenoic acid; Arachidonate | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
| 3DMet | B00061 | ||
Beilstein Reference | 1713889 | ||
ChEBI |
| ||
ChEMBL |
| ||
ChemSpider |
| ||
DrugBank |
| ||
ECHA InfoCard | 100.007.304 | ||
EC Number | 208-033-4 | ||
IUPHAR/BPS |
| ||
KEGG |
| ||
MeSH | Arachidonic+acid | ||
PubChem CID |
| ||
RTECS number | CE6675000 | ||
UNII |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | C20H32O2 | ||
Molar mass | 7002304474000000000♠304.474 g·mol−1 | ||
Density | 0.922 g/cm3 | ||
Melting point | −49 °C (−56 °F; 224 K) | ||
Boiling point | 169 to 171 °C (336 to 340 °F; 442 to 444 K) at 0.15 mmHg | ||
log P | 6.994 | ||
Acidity (pKa) | 4.752 | ||
| Hazards | |||
R-phrases (outdated) | R19 | ||
NFPA 704 |  1 1 0 | ||
Flash point | 113 °C (235 °F; 386 K) | ||
| Related compounds | |||
Related compounds | Eicosatetraenoic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6),[citation needed] or 20:4(5,8,11,14).[2] It is structurally related to the saturated arachidic acid found in cupuaçu butter (L. arachis – peanut).[3]
Contents
1 Chemistry
2 Biology
3 Conditionally essential fatty acid
4 Synthesis and cascade in humans
4.1 PLA2 activation
4.2 PLC activation
5 In the body
5.1 Muscle growth
5.2 Brain
5.3 Alzheimer's disease
5.4 Bodybuilding supplement
6 Dietary arachidonic acid and inflammation
7 Health effects of arachidonic acid supplementation
8 See also
9 References
10 External links
Chemistry
![]()
In chemical structure, arachidonic acid is a carboxylic acid with a 20-carbon chain and four cis-double bonds; the first double bond is located at the sixth carbon from the omega end.
Some chemistry sources define 'arachidonic acid' to designate any of the eicosatetraenoic acids. However, almost all writings in biology, medicine, and nutrition limit the term to all cis-5,8,11,14-eicosatetraenoic acid.
Biology
Arachidonic acid is a polyunsaturated fatty acid present in the phospholipids (especially phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositides) of membranes of the body's cells, and is abundant in the brain, muscles, and liver. Skeletal muscle is an especially active site of arachidonic acid retention, accounting for roughly 10-20% of the phospholipid fatty acid content typically.[4]
In addition to being involved in cellular signaling as a lipid second messenger involved in the regulation of signaling enzymes, such as PLC-γ, PLC-δ, and PKC-α, -β, and -γ isoforms, arachidonic acid is a key inflammatory intermediate and can also act as a vasodilator.[5] (Note separate synthetic pathways, as described in section below.)
Conditionally essential fatty acid

Arachidonic acid in the human body usually comes from dietary animal sources (meat, eggs) or is synthesized from linoleic acid.
Arachidonic acid is not one of the essential fatty acids. However, it does become essential if a deficiency in linoleic acid exists or if there is an inability to convert linoleic acid to arachidonic acid occurs.
Some mammals lack the ability or have a very limited capacity to convert linoleic acid to arachidonic acid, making it an essential part of their diets. Since little or no arachidonic acid is found in common plants, such animals are obligate carnivores; the cat is a common example having inability to desaturate essential fatty acids.[6][7] A commercial source of arachidonic acid has been derived, however, from the fungus Mortierella alpina.[8]
Synthesis and cascade in humans
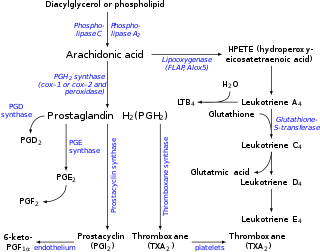
Eicosanoid synthesis.
Arachidonic acid is freed from a phospholipid molecule by the enzyme phospholipase A2 (PLA2), which cleaves off the fatty acid, but can also be generated from DAG by diacylglycerol lipase.[5]
Arachidonic acid generated for signaling purposes appears to be derived by the action of group IVA cytosolic phospholipase A2 (cPLA2, 85 kDa), whereas inflammatory arachidonic acid is generated by the action of a low-molecular-weight secretory PLA2 (sPLA2, 14-18 kDa).[5]
Arachidonic acid is the precursor that is metabolized by various enzymes to a wide range of biologically and clinically important eicosanoids and metabolites of these eicosanoids:
- The enzymes cyclooxygenase-1 and -2 (i.e. prostaglandin G/H synthase 1 and 2 {PTGS1 and PTGS2}) metabolize arachidonic acid to prostaglandin G2 and prostaglandin H2, which in turn may be converted to various prostaglandins, to prostacyclin, to thromboxanes, and to the 17-carbon product of thromboxane metabolism of prostaglandin G2/H2, 12-Hydroxyheptadecatrienoic acid (12-HHT).[9][10]
- The enzyme 5-lipoxygenase metabolizes arachidonic acid to 5-hydroperoxyeicosatetraenoic acid (5-HPETE), which in turn is metabolized to various leukotrienes (i.e. leukotriene B4, leukotriene C4, leukotriene D4, and leukotriene E4 as well as to 5-hydroxyeicosatetraenoic acid (5-HETE) which may then be further metabolized to 5-HETE's more potent 5-keto analog, 5-oxo-eicosatetraenoic acid (5-oxo-ETE) (also see 5-Hydroxyeicosatetraenoic acid.[11]
- The enzymes 15-lipoxygenase-1 (ALOX15 and 15-lipoxygenase-2 (ALOX15B metabolize arachidonic acid to 15-hydroperoxyeicosatetraenoic acid (15-HPETE) which may then be further metabolized to 15-hydroxyeicosatetraenoic acid (15-HETE) and lipoxins;[12][13][14] 15-Lipoxygenase-1 may also further metabolize 15-HPETE to eoxins in a pathway analogous to (and presumably using the same enzymes as used in) the pathway which metabolizes 5-HPETE to leukotrienes.[15]
- The enzyme 12-lipoxygenase (ALOX12) metabolizes arachidonic acid to 12-hydroperoxyeicosatetraenoic acid (12-HPETE) which may then be metabolized to 12-hydroxyeicosatetraenoic acid (12-HETE) and to hepoxilins.[16]
- Arachidonic acid is also used in the biosynthesis of anandamide.[17]
- Some arachidonic acid is converted into hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids (EETs) by epoxygenase.[18]
The production of these derivatives and their actions in the body are collectively known as the "arachidonic acid cascade"; see essential fatty acid interactions and the enzyme and metabolite linkages given in the previous paragraph for more details.
PLA2 activation
PLA2, in turn, is activated by ligand binding to receptors, including:
5-HT2 receptors [19]
mGLUR1[19]
bFGF receptor[19]
IFN-α receptor[19]
IFN-γ receptor[19]
Furthermore, any agent increasing intracellular calcium may cause activation of some forms of PLA2.[20]
PLC activation
Alternatively, arachidonic acid may be cleaved from phospholipids after phospholipase C (PLC) cleaves off the inositol trisphosphate group, yielding diacylglycerol (DAG), which subsequently is cleaved by DAG lipase to yield arachidonic acid.[19]
Receptors that activate this pathway include:
A1 receptor[20]
D2 receptor[20]
α-2 adrenergic receptor[20]
5-HT1 receptor[20]
PLC may also be activated by MAP kinase. Activators of this pathway include PDGF and FGF.[20]
In the body
Muscle growth
Arachidonic acid promotes the repair and growth of skeletal muscle tissue via conversion to prostaglandin PGF2alpha during and following physical exercise.[21] PGF2alpha promotes muscle protein synthesis by signaling through the Akt/mTOR pathway,[21] similar to leucine, β-hydroxy β-methylbutyric acid, and phosphatidic acid.
Brain
Arachidonic acid is one of the most abundant fatty acids in the brain, and is present in similar quantities to docosahexaenoic acid (DHA). The two account for about 20% of its fatty-acid content.[22] Like DHA, neurological health is reliant upon sufficient levels of arachidonic acid. Among other things, arachidonic acid helps to maintain hippocampal cell membrane fluidity.[23] It also helps protect the brain from oxidative stress by activating peroxisome proliferator-activated receptor gamma.[24] ARA also activates syntaxin-3 (STX-3), a protein involved in the growth and repair of neurons.[25]
Arachidonic acid is also involved in early neurological development. In one study, infants (18 months) given supplemental arachidonic acid for 17 weeks demonstrated significant improvements in intelligence, as measured by the Mental Development Index.[26] This effect is further enhanced by the simultaneous supplementation of ARA with DHA.
In adults, the disturbed metabolism of ARA may contribute to neuropsychiatric disorders such as Alzheimer's disease and bipolar disorder.[27] There is evidence of significant alterations in the conversion of arachidonic acid to other bioactive molecules (overexpression or disturbances in the ARA enzyme cascade) in these conditions.
Alzheimer's disease
Studies on arachidonic acid and the pathogenesis of Alzheimer's disease is mixed, with one study of AA and its metabolites that suggests they are associated with the onset of Alzheimer's disease,[28] whereas another study suggests that the supplementation of arachidonic acid during the early stages of this disease may be effective in reducing symptoms and slowing the disease progress.[29] Additional studies on arachidonic acid supplementation for Alzheimer's patients are needed. Another study indicates that air pollution is the source of inflammation and arachidonic acid metabolites promote the inflammation to signal the immune system of the cell damage.[30]
Bodybuilding supplement
Arachidonic acid is marketed as an anabolic bodybuilding supplement in a variety of products. Supplementation of arachidonic acid (1,500 mg/day for 8 weeks) has been shown to increase lean body mass, strength, and anaerobic power in experienced resistance-trained men. This was demonstrated in a placebo-controlled study at the University of Tampa. Thirty men (aged 20.4 ± 2.1 years) took arachidonic acid or a placebo for 8 weeks, and participated in a controlled resistance-training program. After 8 weeks, lean body mass (LBM) had increased significantly, and to a greater extent, in the ARA group (1.62 kg) vs. placebo (0.09 kg) (p<0.05). The change in muscle thickness was also greater in the ARA group (.47 cm) than placebo (.25 cm) (p<0.05). Wingate anaerobic power increased to a greater extent in ARA group as well (723.01 to 800.66 W) vs. placebo (738.75 to 766.51 W). Lastly, the change in total strength was significantly greater in the ARA group (109.92 lbs.) compared to placebo (75.78 lbs.). These results suggest that ARA supplementation can positively augment adaptations in strength and skeletal muscle hypertrophy in resistance-trained men.[31]
An earlier clinical study examining the effects of 1,000 mg/day of arachidonic acid for 50 days found supplementation to enhance anaerobic capacity and performance in exercising men. During this study, a significant group–time interaction effect was observed in Wingate relative peak power (AA: 1.2 ± 0.5; P: -0.2 ± 0.2 W•kg-1, p=0.015). Statistical trends were also seen in bench press 1RM (AA: 11.0 ± 6.2; P: 8.0 ± 8.0 kg, p=0.20), Wingate average power (AA:37.9 ± 10.0; P: 17.0 ± 24.0 W, p=0.16), and Wingate total work (AA: 1292 ± 1206; P: 510 ± 1249 J, p=0.087). AA supplementation during resistance training promoted significant increases in relative peak power with other performance-related variables approaching significance. These findings support the use of AA as an ergogenic.[32]
Dietary arachidonic acid and inflammation
Increased consumption of arachidonic acid will not cause inflammation during normal metabolic conditions unless lipid peroxidation products are mixed in. Arachidonic acid is metabolized to both proinflammatory and anti-inflammatory eicosanoids during and after the inflammatory response, respectively. Arachidonic acid is also metabolized to inflammatory and anti-inflammatory eicosanoids during and after physical activity to promote growth. However, chronic inflammation from exogenous toxins and excessive exercise should not be confused with acute inflammation from exercise and sufficient rest that is required by the inflammatory response to promote the repair and growth of the micro tears of tissues.[33] However, the evidence is mixed. Some studies giving between 840 mg and 2,000 mg per day to healthy individuals for up to 50 days have shown no increases in inflammation or related metabolic activities.[33][34][35][36] However, others show that increased arachidonic acid levels are actually associated with reduced pro-inflammatory IL-6 and IL-1 levels and increased anti-inflammatory tumor necrosis factor-beta.[37] This may result in a reduction in systemic inflammation.[medical citation needed]
Arachidonic acid does still play a central role in inflammation related to injury and many diseased states. How it is metabolized in the body dictates its inflammatory or anti-inflammatory activity. Individuals suffering from joint pains or active inflammatory disease may find that increased arachidonic acid consumption exacerbates symptoms, presumably because it is being more readily converted to inflammatory compounds.[medical citation needed] Likewise, high arachidonic acid consumption is not advised for individuals with a history of inflammatory disease, or who are in compromised health. Of note, while ARA supplementation does not appear to have proinflammatory effects in healthy individuals, it may counter the anti-inflammatory effects of omega-3 fatty acid supplementation.[38]
Health effects of arachidonic acid supplementation
Arachidonic acid supplementation in daily doses of 1,000–1,500 mg for 50 days has been well tolerated during several clinical studies, with no significant side effects reported. All common markers of health, including kidney and liver function,[35] serum lipids,[39] immunity,[40] and platelet aggregation[34] appear to be unaffected with this level and duration of use. Furthermore, higher concentrations of ARA in muscle tissue may be correlated with improved insulin sensitivity.[41] Arachidonic acid supplementation of the diets of healthy adults appears to offer no toxicity or significant safety risk.
While studies looking at arachidonic acid supplementation in sedentary subjects have failed to find changes in resting inflammatory markers in doses up to 1,500 mg daily, strength-trained subjects may respond differently. One study reported a significant reduction in resting inflammation (via marker IL-6) in young men supplementing 1,000 mg/day of arachidonic acid for 50 days in combination with resistance training. This suggests that rather being pro-inflammatory, supplementation of ARA while undergoing resistance training may actually improve the regulation of systemic inflammation.[42]
A meta-analysis looking for associations between heart disease risk and individual fatty acids reported a significantly reduced risk of heart disease with higher levels of EPA and DHA (omega-3 fats), as well as the omega-6 arachidonic acid.[43] A scientific advisory from the American Heart Association has also favorably evaluated the health impact of dietary omega-6 fats, including arachidonic acid.[33] The group does not recommend limiting this essential fatty acid. In fact, the paper recommends individuals follow a diet that consists of at least 5–10% of calories coming from omega-6 fats, including arachidonic acid. It suggests dietary ARA is not a risk factor for heart disease, and may play a role in maintaining optimal metabolism and reduced heart disease risk. Maintaining sufficient intake levels of both omega-3 and omega-6 fatty acids, therefore, is recommended for optimal health.
Arachidonic acid is not carcinogenic, and studies show dietary level is not associated (positively or negatively) with risk of cancers.[44][45][46][47] ARA remains integral to the inflammatory and cell growth process, however, which is disturbed in many types of disease including cancer. Therefore, the safety of arachidonic acid supplementation in patients suffering from cancer, inflammatory, or other diseased states is unknown, and supplementation is not recommended.
See also
Aspirin—inhibits cyclooxygenase enzyme, preventing conversion of arachidonic acid to other signal molecules- Fish oil
- Polyunsaturated fat
- Polyunsaturated fatty acid
References
^ Pubchem. "5,8,11,14-Eicosatetraenoic acid | C20H32O2 - PubChem". pubchem.ncbi.nlm.nih.gov. Retrieved 2016-03-31..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "IUPAC Lipid nomenclature: Appendix A: names of and symbols for higher fatty acids". www.sbcs.qmul.ac.uk.
^ "Dorland's Medical Dictionary – 'A'". Archived from the original on 11 January 2007. Retrieved 2007-01-12.
^ Smith, GI; Atherton, P; Reeds, DN; Mohammed, BS; Rankin, D; Rennie, MJ; Mittendorfer, B (Sep 2011). "Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women". Clinical Science. 121 (6): 267–78. doi:10.1042/cs20100597. PMC 3499967. PMID 21501117.
^ abc Baynes, John W.; Marek H. Dominiczak (2005). Medical Biochemistry 2nd. Edition. Elsevier Mosby. p. 555. ISBN 0-7234-3341-0.
^ MacDonald, ML; Rogers, QR; Morris, JG (1984). "Nutrition of the Domestic Cat, a Mammalian Carnivore". Annual Review of Nutrition. 4: 521–62. doi:10.1146/annurev.nu.04.070184.002513. PMID 6380542.
^ Rivers, JP; Sinclair, AJ; Craqford, MA (1975). "Inability of the cat to desaturate essential fatty acids". Nature. 258 (5531): 171–3. Bibcode:1975Natur.258..171R. doi:10.1038/258171a0. PMID 1186900.
^ Production of life'sARA™, www.lifesdha.com/
^ Wlodawer, P; Samuelsson, B (1973). "On the organization and mechanism of prostaglandin synthetase". The Journal of Biological Chemistry. 248 (16): 5673–8. PMID 4723909.
^ Smith, W. L.; Song, I (2002). "The enzymology of prostaglandin endoperoxide H synthases-1 and -2". Prostaglandins & other lipid mediators. 68–69: 115–28. doi:10.1016/s0090-6980(02)00025-4. PMID 12432913.
^ Powell, W. S.; Rokach, J (Apr 2015). "Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid". Biochim Biophys Acta. 1851 (4): 340–355. doi:10.1016/j.bbalip.2014.10.008. PMID 25449650.
^ Brash, A. R.; Boeglin, W. E.; Chang, M. S. (Jun 1997). "Discovery of a second 15S-lipoxygenase in humans". Proc Natl Acad Sci U S A. 94 (12): 6148–52. Bibcode:1997PNAS...94.6148B. doi:10.1073/pnas.94.12.6148. PMC 21017. PMID 9177185.
^ Zhu, D; Ran, Y (May 2012). "Role of 15-lipoxygenase/15-hydroxyeicosatetraenoic acid in hypoxia-induced pulmonary hypertension". J Physiol Sci. 62 (3): 163–72. doi:10.1007/s12576-012-0196-9. PMID 22331435.
^ Romano, M; Cianci, E; Simiele, F; Recchiuti, A (Aug 2015). "Lipoxins and aspirin-triggered lipoxins in resolution of inflammation". Eur J Pharmacol. 760: 49–63. doi:10.1016/j.ejphar.2015.03.083. PMID 25895638.
^ Feltenmark, S; Gautam, N; Brunnström, A; Griffiths, W; Backman, L; Edenius, C; Lindbom, L; Björkholm, M; Claesson, H. E. (Jan 2008). "Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells". Proc Natl Acad Sci U S A. 105 (2): 680–5. Bibcode:2008PNAS..105..680F. doi:10.1073/pnas.0710127105. PMC 2206596. PMID 18184802.
^ Porro, B; Songia, P; Squellerio, I; Tremoli, E; Cavalca, V (Aug 2014). "Analysis, physiological and clinical significance of 12-HETE: A neglected platelet-derived 12-lipoxygenase product". J Chromatogr B. 964: 26–40. doi:10.1016/j.jchromb.2014.03.015. PMID 24685839.
^ Ueda, Natsuo; Tsuboi, Kazuhito; Uyama, Toru (May 2013). "Metabolism of endocannabinoids and related N -acylethanolamines: Canonical and alternative pathways". FEBS J. 280 (9): 1874–94. doi:10.1111/febs.12152. PMID 23425575.
^ Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 108. ISBN 1-4160-2328-3.
^ abcdef Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 103. ISBN 1-4160-2328-3.
^ abcdef Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 104. ISBN 1-4160-2328-3.
^ ab Trappe TA, Liu SZ (2013). "Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise". J. Appl. Physiol. 115 (6): 909–19. doi:10.1152/japplphysiol.00061.2013. PMC 3764617. PMID 23539318.
^ Crawford, MA; Sinclair, AJ (1971). "Nutritional influences in the evolution of mammalian brain. In: lipids, malnutrition & the developing brain". Ciba Foundation symposium: 267–92. PMID 4949878.
^ Fukaya, T.; Gondaira, T.; Kashiyae, Y.; Kotani, S.; Ishikura, Y.; Fujikawa, S.; Kiso, Y.; Sakakibara, M. (2007). "Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats". Neurobiology of Aging. 28 (8): 1179–1186. doi:10.1016/j.neurobiolaging.2006.05.023. PMID 16790296.
^ Wang, ZJ; Liang, CL; Li, GM; Yu, CY; Yin, M (2006). "Neuroprotective effects of arachidonic acid against oxidative stress on rat hippocampal slices". Chemico-Biological Interactions. 163 (3): 207–17. doi:10.1016/j.cbi.2006.08.005. PMID 16982041.
^ Darios, F; Davletov, B (2006). "Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3". Nature. 440 (7085): 813–7. Bibcode:2006Natur.440..813D. doi:10.1038/nature04598. PMID 16598260.
^ Birch, Eileen E; Garfield, Sharon; Hoffman, Dennis R; Uauy, Ricardo; Birch, David G (2007). "A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants". Developmental Medicine & Child Neurology. 42 (3): 174–181. doi:10.1111/j.1469-8749.2000.tb00066.x.
^ Rapoport, SI (2008). "Arachidonic acid and the brain". The Journal of Nutrition. 138 (12): 2515–20. PMC 3415870. PMID 19022981.
^ Amtul, Z.; Uhrig, M.; Wang, L.; Rozmahel, R. F.; Beyreuther, K. (2012). "Detrimental effects of arachidonic acid and its metabolites in cellular and mouse models of Alzheimer's disease: Structural insight". Neurobiology of Aging. 33 (4): 831.e21–31. doi:10.1016/j.neurobiolaging.2011.07.014. PMID 21920632.
^ Schaeffer, EL; Forlenza, OV; Gattaz, WF (2009). "Phospholipase A2 activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease". Psychopharmacology. 202 (1–3): 37–51. doi:10.1007/s00213-008-1351-0. PMID 18853146.
^ Calderón-Garcidueñas, L; Reed, W; Maronpot, R. R.; Henríquez-Roldán, C; Delgado-Chavez, R; Calderón-Garcidueñas, A; Dragustinovis, I; Franco-Lira, M; Aragón-Flores, M; Solt, A. C.; Altenburg, M; Torres-Jardón, R; Swenberg, J. A. (2004). "Brain inflammation and Alzheimer's-like pathology in individuals exposed to severe air pollution". Toxicologic Pathology. 32 (6): 650–8. doi:10.1080/01926230490520232. PMID 15513908.
^ Ormes, Jacob. "Effects of Arachidonic Acid Supplementation on Skeletal Muscle Mass, Strength, and Power". NSCA ePoster Gallery. National Strength and Conditioning Association.
^ Roberts, MD; Iosia, M; Kerksick, CM; Taylor, LW; Campbell, B; Wilborn, CD; Harvey, T; Cooke, M; Rasmussen, C; Greenwood, Mike; Wilson, Ronald; Jitomir, Jean; Willoughby, Darryn; Kreider, Richard B (2007). "Effects of arachidonic acid supplementation on training adaptations in resistance-trained males". Journal of the International Society of Sports Nutrition. 4: 21. doi:10.1186/1550-2783-4-21. PMC 2217562. PMID 18045476.
^ abc Harris, WS; Mozaffarian, D; Rimm, E; Kris-Etherton, P; Rudel, LL; Appel, LJ; Engler, MM; Engler, MB; Sacks, F (2009). "Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention". Circulation. 119 (6): 902–7. doi:10.1161/CIRCULATIONAHA.108.191627. PMID 19171857.
^ ab Nelson, GJ; Schmidt, PC; Bartolini, G; Kelley, DS; Kyle, D (1997). "The effect of dietary arachidonic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans". Lipids. 32 (4): 421–5. doi:10.1007/s11745-997-0055-7. PMID 9113631.
^ ab Changes in whole blood and clinical safety markers over 50 days of concomitant arachidonic acid supplementation and resistance training. Wilborn, C, M Roberts, C Kerksick, M Iosia, L Taylor, B Campbell, T Harvey, R Wilson, M. Greenwood, D Willoughby and R Kreider. Proceedings of the International Society of Sports Nutrition (ISSN) Conference June 15–17, 2006.
^ Pantaleo, P; Marra, F; Vizzutti, F; Spadoni, S; Ciabattoni, G; Galli, C; La Villa, G; Gentilini, P; Laffi, G (2004). "Effects of dietary supplementation with arachidonic acid on platelet and renal function in patients with cirrhosis". Clinical Science. 106 (1): 27–34. doi:10.1042/CS20030182. PMID 12877651.
^ Ferrucci, L; Cherubini, A; Bandinelli, S; Bartali, B; Corsi, A; Lauretani, F; Martin, A; Andres-Lacueva, C; Senin, U; Guralnik, JM (2006). "Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers". The Journal of Clinical Endocrinology and Metabolism. 91 (2): 439–46. doi:10.1210/jc.2005-1303. PMID 16234304.
^ Li, B; Birdwell, C; Whelan, J (1994). "Antithetic relationship of dietary arachidonic acid and eicosapentaenoic acid on eicosanoid production in vivo". Journal of Lipid Research. 35 (10): 1869–77. PMID 7852864.
^ Nelson, GJ; Schmidt, PC; Bartolini, G; Kelley, DS; Phinney, SD; Kyle, D; Silbermann, S; Schaefer, EJ (1997). "The effect of dietary arachidonic acid on plasma lipoprotein distributions, apoproteins, blood lipid levels, and tissue fatty acid composition in humans". Lipids. 32 (4): 427–33. doi:10.1007/s11745-997-0056-6. PMID 9113632.
^ Kelley, DS; Taylor, PC; Nelson, GJ; MacKey, BE (1998). "Arachidonic acid supplementation enhances synthesis of eicosanoids without suppressing immune functions in young healthy men". Lipids. 33 (2): 125–30. doi:10.1007/s11745-998-0187-9. PMID 9507233.
^ Borkman, M; Storlien, LH; Pan, DA; Jenkins, AB; Chisholm, DJ; Campbell, LV (1993). "The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids". The New England Journal of Medicine. 328 (4): 238–44. doi:10.1056/NEJM199301283280404. PMID 8418404.
^ Roberts, MD; Iosia, M; Kerksick, CM; Taylor, LW; Campbell, B; Wilborn, CD; Harvey, T; Cooke, M; Rasmussen, C; Greenwood, M; Wilson, R; Jitomir, J; Willoughby, D; Kreider, RB (Nov 28, 2007). "Effects of arachidonic acid supplementation on training adaptations in resistance-trained males". Journal of the International Society of Sports Nutrition. 4: 21. doi:10.1186/1550-2783-4-21. PMC 2217562. PMID 18045476.
^ Chowdhury, R; Warnakula, S; Kunutsor, S; Crowe, F; Ward, HA; Johnson, L; Franco, OH; Butterworth, AS; Forouhi, NG; Thompson, SG; Khaw, KT; Mozaffarian, D; Danesh, J; Di Angelantonio, E (Mar 18, 2014). "Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis". Annals of Internal Medicine. 160 (6): 398–406. doi:10.7326/M13-1788. PMID 24723079.
^ Schuurman, AG; Van Den Brandt, PA; Dorant, E; Brants, HA; Goldbohm, RA (1999). "Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study". Cancer. 86 (6): 1019–27. doi:10.1002/(SICI)1097-0142(19990915)86:6<1019::AID-CNCR18>3.0.CO;2-H. PMID 10491529.
^ Leitzmann, MF; Stampfer, MJ; Michaud, DS; Augustsson, K; Colditz, GC; Willett, WC; Giovannucci, EL (2004). "Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer". The American Journal of Clinical Nutrition. 80 (1): 204–16. PMID 15213050.
^ Astorg, P (2005). "Dietary fatty acids and colorectal and prostate cancers: epidemiological studies". Bulletin du cancer. 92 (7): 670–84. PMID 16123006.
^ Whelan, J; McEntee, MF (2004). "Dietary (n-6) PUFA and intestinal tumorigenesis". The Journal of Nutrition. 134 (12 Suppl): 3421S–3426S. PMID 15570048.
External links
Arachidonic Acid at acnp.org
Arachidonic+Acid at the US National Library of Medicine Medical Subject Headings (MeSH)
^ Sun, Y. and Bennett, A. [1], "Cannabinoids: A New Group of Agonists of PPARs" PPAR Research, 2007; 2007: 23513. doi: 10.1155/2007/23513
^ O'Sullivan, S.E. [2], "An update on PPAR activation by cannabinoids", British Journal of Pharmacology, Volume 173, Issue 12, Pages 1899-1910. https://doi.org/10.1111/bph.13497