Staining

A stained histologic specimen, sandwiched between a glass microscope slide and coverslip, mounted on the stage of a light microscope.
Staining is an auxiliary technique used in microscopy to enhance contrast in the microscopic image. Stains and dyes are frequently used in biology and medicine to highlight structures in biological tissues for viewing, often with the aid of different microscopes. Stains may be used to define and examine bulk tissues (highlighting, for example, muscle fibers or connective tissue), cell populations (classifying different blood cells, for instance), or organelles within individual cells.
In biochemistry it involves adding a class-specific (DNA, proteins, lipids, carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescent tagging can serve similar purposes. Biological staining is also used to mark cells in flow cytometry, and to flag proteins or nucleic acids in gel electrophoresis.
Simple staining is staining with only one stain/dye. There are various kinds of multiple staining, many of which are examples of counterstaining, differential staining, or both, including double staining and triple staining.
Staining is not limited to biological materials, it can also be used to study the morphology of other materials for example the lamellar structures of semi-crystalline polymers or the domain structures of block copolymers.
Contents
1 In vivo vs In vitro
1.1 In vitro methods
1.1.1 Preparation
1.1.2 Standardization
1.1.3 Negative staining
1.2 Specific techniques
1.2.1 Gram staining
1.2.2 Endospore staining
1.2.3 Ziehl-Neelsen stain
1.2.4 Haematoxylin and eosin (H&E) staining
1.2.5 Papanicolaou staining
1.2.6 PAS staining
1.2.7 Masson's trichrome
1.2.8 Romanowsky stains
1.2.9 Silver staining
1.2.10 Sudan staining
1.2.11 Conklin's staining
2 Common biological stains
2.1 Acridine orange
2.2 Bismarck brown
2.3 Carmine
2.4 Coomassie blue
2.5 Cresyl violet
2.6 Crystal violet
2.7 DAPI
2.8 Eosin
2.9 Ethidium bromide
2.10 Acid fuchsine
2.11 Haematoxylin
2.12 Hoechst stains
2.13 Iodine
2.14 Malachite green
2.15 Methyl green
2.16 Methylene blue
2.17 Neutral red
2.18 Nile blue
2.19 Nile red
2.20 Osmium tetroxide (formal name: osmium tetraoxide)
2.21 Propidium Iodide
2.22 Rhodamine
2.23 Safranine
3 Stainability of tissues
4 Electron microscopy
4.1 Phosphotungstic acid
4.2 Osmium tetroxide
4.3 Ruthenium tetroxide
5 See also
6 References
7 Further reading
8 External links
In vivo vs In vitro
In vivo staining (also called vital staining or intravital staining) is the process of dyeing living tissues—in vivo means "in life" (compare with in vitro staining). By causing certain cells or structures to take on contrasting colour(s), their form (morphology) or position within a cell or tissue can be readily seen and studied. The usual purpose is to reveal cytological details that might otherwise not be apparent; however, staining can also reveal where certain chemicals or specific chemical reactions are taking place within cells or tissues.
In vitro staining involves colouring cells or structures that have been removed from their biological context. Certain stains are often combined to reveal more details and features than a single stain alone. Combined with specific protocols for fixation and sample preparation, scientists and physicians can use these standard techniques as consistent, repeatable diagnostic tools. A counterstain is stain that makes cells or structures more visible, when not completely visible with the principal stain.
- For example, crystal violet stains only Gram-positive bacteria in Gram staining. A safranin counterstain is applied that stains all cells, allowing identification of Gram-negative bacteria.
While ex vivo, many cells continue to live and metabolize until they are "fixed". Some staining methods are based on this property. Those stains excluded by the living cells but taken up by the already dead cells are called vital stains (e.g. trypan blue or propidium iodide for eukaryotic cells). Those that enter and stain living cells are called supravital stains (e.g. New Methylene Blue and brilliant cresyl blue for reticulocyte staining). However, these stains are eventually toxic to the organism, some more so than others. Partly due to their toxic interaction inside a living cell, when supravital stains enter a living cell, they might produce a characteristic pattern of staining different from the staining of an already fixed cell (e.g. "reticulocyte" look versus diffuse "polychromasia"). To achieve desired effects, the stains are used in very dilute solutions ranging from 1:5000 to 1:500000 (Howey, 2000). Note that many stains may be used in both living and fixed cells.
In vitro methods
Preparation
The preparatory steps involved depend on the type of analysis planned; some or all of the following procedures may be required.
Fixation–which may itself consist of several steps–aims to preserve the shape of the cells or tissue involved as much as possible. Sometimes heat fixation is used to kill, adhere, and alter the specimen so it accepts stains. Most chemical fixatives (chemicals causing fixation) generate chemical bonds between proteins and other substances within the sample, increasing their rigidity. Common fixatives include formaldehyde, ethanol, methanol, and/or picric acid. Pieces of tissue may be embedded in paraffin wax to increase their mechanical strength and stability and to make them easier to cut into thin slices.
Permeabilization involves treatment of cells with (usually) a mild surfactant. This treatment dissolves cell membranes, and allows larger dye molecules into the cell's interior.
Mounting usually involves attaching the samples to a glass microscope slide for observation and analysis. In some cases, cells may be grown directly on a slide. For samples of loose cells (as with a blood smear or a pap smear) the sample can be directly applied to a slide. For larger pieces of tissue, thin sections (slices) are made using a microtome; these slices can then be mounted and inspected.
Standardization
Most of the dyes commonly used in microscopy are available as BSC-certified stains. This means that samples of the manufacturer's batch have been tested by an independent body, the Biological Stain Commission (BSC), and found to meet or exceed certain standards of purity, dye content and performance in staining techniques. These standards are published in the Commission's journal Biotechnic & Histochemistry.[1] Many dyes are inconsistent in composition from one supplier to another. The use of BSC-certified stains eliminates a source of unexpected results.[2]
Some vendors sell stains "certified" by themselves rather than by the Biological Stain Commission. Such products may or may not be suitable for diagnostic and other applications.[3]
Negative staining
A simple staining method for bacteria that is usually successful, even when the "positive staining" methods detailed below fail, is to use a negative stain. This can be achieved by smearing the sample onto the slide and then applying nigrosin (a black synthetic dye) or India ink (an aqueous suspension of carbon particles). After drying, the microorganisms may be viewed in bright field microscopy as lighter inclusions well-contrasted against the dark environment surrounding them.[4] Note: negative staining is a mild technique that may not destroy the microorganisms, and is therefore unsuitable for studying pathogens.
Specific techniques
Gram staining
Gram staining is used to determine gram status to classify bacteria broadly. It is based on the composition of their cell wall. Gram staining uses crystal violet to stain cell walls, iodine as a mordant, and a fuchsin or safranin counterstain to mark all bacteria. Gram status is important in medicine; the presence or absence of a cell wall changes the bacterium's susceptibility to some antibiotics.
Gram-positive bacteria stain dark blue or violet. Their cell wall is typically rich with peptidoglycan and lacks the secondary membrane and lipopolysaccharide layer found in Gram-negative bacteria.
On most Gram-stained preparations, Gram-negative organisms appear red or pink because they are counterstained. Because of presence of higher lipid content, after alcohol-treatment, the porosity of the cell wall increases, hence the CVI complex (crystal violet – iodine) can pass through. Thus, the primary stain is not retained. Also, in contrast to most Gram-positive bacteria, Gram-negative bacteria have only a few layers of peptidoglycan and a secondary cell membrane made primarily of lipopolysaccharide.
Endospore staining
Endospore staining is used to identify the presence or absence of endospores, which make bacteria very difficult to kill. This is particularly useful for identifying endospore-forming bacterial pathogens such as Clostridium difficile.
Ziehl-Neelsen stain
Ziehl-Neelsen staining is used to stain species of Mycobacterium tuberculosis that do not stain with the standard laboratory staining procedures such as Gram staining.
The stains used are the red coloured Carbol fuchsin that stains the bacteria and a counter stain such as Methylene blue
Haematoxylin and eosin (H&E) staining
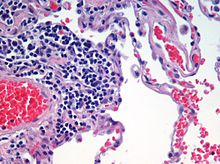
Microscopic view of a histologic specimen of human lung tissue stained with hematoxylin and eosin.
Haematoxylin and eosin staining protocol is used frequently in histology to examine thin sections of tissue. Haematoxylin stains cell nuclei blue, while eosin stains cytoplasm, connective tissue and other extracellular substances pink or red. Eosin is strongly absorbed by red blood cells, colouring them bright red. In a skilfully made H & E preparation the red blood cells are almost orange, and collagen and cytoplasm (especially muscle) acquire different shades of pink. When the staining is done by a machine, the subtle differences in eosinophilia are often lost. Hematoxylin stains the cell nucleus and other acidic structures (such as RNA-rich portions of the cytoplasm and the matrix of hyaline cartilage) blue. In contrast, eosin stains the cytoplasm and collagen pink.
Papanicolaou staining
Papanicolaou staining, or Pap staining, is a frequently used method for examining cell samples from various bodily secretions. It is frequently used to stain Pap smear specimens. It uses a combination of haematoxylin, Orange G, eosin Y, Light Green SF yellowish, and sometimes Bismarck Brown Y.
PAS staining

PAS diastase showing the fungus Histoplasma.
Periodic acid-Schiff staining is used to mark carbohydrates (glycogen, glycoprotein, proteoglycans). It is used to distinguish different types of glycogen storage diseases.
Masson's trichrome
Masson's trichrome is (as the name implies) a three-colour staining protocol. The recipe has evolved from Masson's original technique for different specific applications, but all are well-suited to distinguish cells from surrounding connective tissue. Most recipes produce red keratin and muscle fibers, blue or green staining of collagen and bone, light red or pink staining of cytoplasm, and black cell nuclei.
Romanowsky stains
The Romanowsky stains are all based on a combination of eosinate (chemically reduced eosin) and methylene blue (sometimes with its oxidation products azure A and azure B). Common variants include Wright's stain, Jenner's stain, May-Grunwald stain, Leishman stain and Giemsa stain.
All are used to examine blood or bone marrow samples. They are preferred over H&E for inspection of blood cells because different types of leukocytes (white blood cells) can be readily distinguished. All are also suited to examination of blood to detect blood-borne parasites such as malaria.
Silver staining

Gömöri methenamine silver stain demonstrating histoplasma (black round balls).
Silver staining is the use of silver to stain histologic sections. This kind of staining is important especially to show proteins (for example type III collagen) and DNA. It is used to show both substances inside and outside cells. Silver staining is also used in temperature gradient gel electrophoresis.
Some cells are argentaffin. These reduce silver solution to metallic silver after formalin fixation. This method was discovered by Italian Camillo Golgi, by using a reaction between silver nitrate and potassium dichromate, thus precipitating silver chromate in some cells (see Golgi's method). Other cells are argyrophilic. These reduce silver solution to metallic silver after being exposed to the stain that contains a reductant, for example hydroquinone or formalin.
Sudan staining
Sudan staining is the use of Sudan dyes to stain sudanophilic substances, usually lipids. Sudan III, Sudan IV, Oil Red O, Osmium tetroxide, and Sudan Black B are often used. Sudan staining is often used to determine the level of fecal fat to diagnose steatorrhea.
Conklin's staining
Special technique designed for staining true endospores with the use of malachite green dye, once stained, they do not decolourize.
Common biological stains
Different stains react or concentrate in different parts of a cell or tissue, and these properties are used to advantage to reveal specific parts or areas. Some of the most common biological stains are listed below. Unless otherwise marked, all of these dyes may be used with fixed cells and tissues; vital dyes (suitable for use with living organisms) are noted.
Acridine orange
Acridine orange (AO) is a nucleic acid selective fluorescent cationic dye useful for cell cycle determination. It is cell-permeable, and interacts with DNA and RNA by intercalation or electrostatic attractions. When bound to DNA, it is very similar spectrally to fluorescein. Like fluorescein, it is also useful as a non-specific stain for backlighting conventionally stained cells on the surface of a solid sample of tissue (fluorescence backlighted staining[5]).
Bismarck brown
Bismarck brown (also Bismarck brown Y or Manchester brown) imparts a yellow colour to acid mucins.
Carmine

Carmine staining of a parasitic flatworm.
Carmine is an intensely red dye used to stain glycogen, while Carmine alum is a nuclear stain. Carmine stains require the use of a mordant, usually aluminum.
Coomassie blue
Coomassie blue (also brilliant blue) nonspecifically stains proteins a strong blue colour. It is often used in gel electrophoresis.
Cresyl violet
Cresyl violet stains the acidic components of the neuronal cytoplasm a violet colour, specifically nissl bodies. Often used in brain research.
Crystal violet
Crystal violet, when combined with a suitable mordant, stains cell walls purple. Crystal violet is the stain used in Gram staining.
DAPI
DAPI is a fluorescent nuclear stain, excited by ultraviolet light and showing strong blue fluorescence when bound to DNA. DAPI binds with A=T rich repeats of chromosomes. DAPI is also not visible with regular transmission microscopy. It may be used in living or fixed cells. DAPI-stained cells are especially appropriate for cell counting.[6]
Eosin
Eosin is most often used as a counterstain to haematoxylin, imparting a pink or red colour to cytoplasmic material, cell membranes, and some extracellular structures. It also imparts a strong red colour to red blood cells. Eosin may also be used as a counterstain in some variants of Gram staining, and in many other protocols. There are actually two very closely related compounds commonly referred to as eosin. Most often used is eosin Y (also known as eosin Y ws or eosin yellowish); it has a very slightly yellowish cast. The other eosin compound is eosin B (eosin bluish or imperial red); it has a very faint bluish cast. The two dyes are interchangeable, and the use of one or the other is more a matter of preference and tradition.
Ethidium bromide
Ethidium bromide intercalates and stains DNA, providing a fluorescent red-orange stain. Although it will not stain healthy cells, it can be used to identify cells that are in the final stages of apoptosis – such cells have much more permeable membranes. Consequently, ethidium bromide is often used as a marker for apoptosis in cells populations and to locate bands of DNA in gel electrophoresis. The stain may also be used in conjunction with acridine orange (AO) in viable cell counting. This EB/AO combined stain causes live cells to fluoresce green whilst apoptotic cells retain the distinctive red-orange fluorescence.
Acid fuchsine
Acid fuchsine may be used to stain collagen, smooth muscle, or mitochondria.
Acid fuchsine is used as the nuclear and cytoplasmic stain in Mallory's trichrome method. Acid fuchsine stains cytoplasm in some variants of Masson's trichrome. In Van Gieson's picro-fuchsine, acid fuchsine imparts its red colour to collagen fibres. Acid fuchsine is also a traditional stain for mitochondria (Altmann's method).
Haematoxylin
Haematoxylin (hematoxylin in North America) is a nuclear stain. Used with a mordant, haematoxylin stains nuclei blue-violet or brown. It is most often used with eosin in H&E (haematoxylin and eosin) staining—one of the most common procedures in histology.
Hoechst stains
Hoechst is a bis-benzimidazole derivative compound that binds to the minor groove of DNA. Often used in fluorescence microscopy for DNA staining, Hoechst stains appear yellow when dissolved in aqueous solutions and emit blue light under UV excitation. There are two major types of Hoechst: Hoechst 33258 and Hoechst 33342. The two compounds are functionally similar, but with a little difference in structure. Hoechst 33258 contains a terminal hydroxyl group and is thus more soluble in aqueous solution, however this characteristics reduces its ability to penetrate the plasma membrane. Hoechst 33342 contains an ethyl substitution on the terminal hydroxyl group (i.e. an ethylether group) making it more hydrophobic for easier plasma membrane passage
Iodine
Iodine is used in chemistry as an indicator for starch. When starch is mixed with iodine in solution, an intensely dark blue colour develops, representing a starch/iodine complex. Starch is a substance common to most plant cells and so a weak iodine solution will stain starch present in the cells. Iodine is one component in the staining technique known as Gram staining, used in microbiology.
Lugol's solution or Lugol's iodine (IKI) is a brown solution that turns black in the presence of starches and can be used as a cell stain, making the cell nuclei more visible. Iodine is also used as a mordant in Gram's staining, it enhances dye to enter through the pore present in the cell wall/membrane.
Malachite green
Malachite green (also known as diamond green B or victoria green B) can be used as a blue-green counterstain to safranin in the Gimenez staining technique for bacteria. It can also be used to directly stain spores.
Methyl green
Methyl green is used commonly with bright-field, as well as fluorescence microscopes [7] to dye the chromatin of cells so that they are more easily viewed.
Methylene blue
Methylene blue is used to stain animal cells, such as human cheek cells, to make their nuclei more observable. Also used to stain the blood film and used in cytology.
Neutral red
Neutral red (or toluylene red) stains Nissl substance red. It is usually used as a counterstain in combination with other dyes.
Nile blue
Nile blue (or Nile blue A) stains nuclei blue. It may be used with living cells.
Nile red
Nile red (also known as Nile blue oxazone) is formed by boiling Nile blue with sulfuric acid. This produces a mix of Nile red and Nile blue. Nile red is a lipophilic stain; it will accumulate in lipid globules inside cells, staining them red. Nile red can be used with living cells. It fluoresces strongly when partitioned into lipids, but practically not at all in aqueous solution.
Osmium tetroxide (formal name: osmium tetraoxide)
Osmium tetraoxide is used in optical microscopy to stain lipids. It dissolves in fats, and is reduced by organic materials to elemental osmium, an easily visible black substance.
Propidium Iodide
Propidium iodide is a fluorescent intercalating agent that can be used to stain cells. Propidium iodide is used as a DNA stain in flow cytometry to evaluate cell viability or DNA content in cell cycle analysis, or in microscopy to visualise the nucleus and other DNA-containing organelles. Propidium Iodide cannot cross the membrane of live cells, making it useful to differentiate necrotic, apoptotic and healthy cells. PI also binds to RNA, necessitating treatment with nucleases to distinguish between RNA and DNA staining
Rhodamine
Rhodamine is a protein specific fluorescent stain commonly used in fluorescence microscopy.
Safranine
Safranine (or Safranine O) is a red cationic dye. It binds to nuclei (DNA) and other tissue polyanions, including glycosaminoglycans in cartilage and mast cells, and components of lignin and plastids in plant tissues.[8] Safranine should not be confused with saffron, an expensive natural dye that is used in some methods to impart a yellow colour to collagen, to contrast with blue and red colours imparted by other dyes to nuclei and cytoplasm in animal (including human) tissues.
The incorrect spelling "safranin" is in common use. The -ine ending is appropriate
for safranine O because this dye is an amine,[9][10][2]
Stainability of tissues
Tissues which take up stains are called chromatic. Chromosomes were so named because of their ability to absorb a violet stain.
Positive affinity for a specific stain may be designated by the suffix -philic. For example, tissues that stain with an azure stain may be referred to as azurophilic. This may also be used for more generalized staining properties, such as acidophilic for tissues that stain by acidic stains (most notably eosin), basophilic when staining in basic dyes, and amphophilic[11] when staining with either acid or basic dyes. In contrast, chromophobic tissues do not take up coloured dye readily.
Electron microscopy
As in light microscopy, stains can be used to enhance contrast in transmission electron microscopy. Electron-dense compounds of heavy metals are typically used.
Phosphotungstic acid
Phosphotungstic acid is a common negative stain for viruses, nerves, polysaccharides, and other biological tissue materials.
Osmium tetroxide
Osmium tetroxide is used in optical microscopy to stain lipids. It dissolves in fats, and is reduced by organic materials to elemental osmium, an easily visible black substance. Because it is a heavy metal that absorbs electrons, it is perhaps the most common stain used for morphology in biological electron microscopy. It is also used for the staining of various polymers for the study of their morphology by TEM. OsO
4 is very volatile and extremely toxic. It is a strong oxidizing agent as the osmium has an oxidation number of +8. It aggressively oxidizes many materials, leaving behind a deposit of non-volatile osmium in a lower oxidation state.
Ruthenium tetroxide
Ruthenium tetroxide is equally volatile and even more aggressive than osmium tetraoxide and able to stain even materials that resist the osmium stain, e.g. polyethylene.
Other chemicals used in electron microscopy staining include:
ammonium molybdate, cadmium iodide, carbohydrazide, ferric chloride, hexamine, indium trichloride, lanthanum nitrate, lead acetate, lead citrate, lead(II) nitrate, periodic acid, phosphomolybdic acid, potassium ferricyanide, potassium ferrocyanide, ruthenium red, silver nitrate, silver proteinate, sodium chloroaurate, thallium nitrate, thiosemicarbazide, uranyl acetate, uranyl nitrate, and vanadyl sulfate.
See also
Cytology: the study of cells
Histology: the study of tissues
Immunohistochemistry: the use of antisera to label specific antigens
Ruthenium(II) tris(bathophenanthroline disulfonate), a protein dye.
Vital stain: stains that do not kill cells
PAGE: separation of protein molecules
Barium enema - a type of in vivo stain that creates contrast in the x-ray part of the light spectrum
References
^ Penney DP, Powers JM, Frank M, Churukian C (2002). "Analysis and testing of biological stains—the Biological Stain Commission Procedures". Biotechnic & Histochemistry. 77 (5–6): 237–275. doi:10.1080/714028210. PMID 12564600..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ ab Horobin, Richard; Kiernan, John, eds. (2002). Conn's Biological Stains: A Handbook of Dyes, Stains and Fluorochromes for Use in Biology and Medicine. Taylor & Francis. ISBN 1859960995.
^ "Vendors List - The Biological Stain Commission". biologicalstaincommission.org. Retrieved 25 March 2018.
^ Clark G (1981) Staining Procedures, 4th ed., Baltimore: Williams & Wilkins, p. 412,
ISBN 0683017071.
^ Wells, J (1988). "A Technique for Staining the Superficial Cells of Plucked Hair Follicles and Other Solid Tissues". Stain Technology. 63 (3).
^ Levenfus, I.: An efficient method for counting DAPI-stained cells using Fiji. Grin: Munich. 2011.
ISBN 978-3-640-86284-9
^ Prieto, Daniel; Aparicio, Gonzalo; Morande, Pablo E.; Zolessi, Flavio R. (27 March 2014). "A fast, low cost, and highly efficient fluorescent DNA labeling method using methyl green". Histochemistry and Cell Biology. 142 (3): 335–345. doi:10.1007/s00418-014-1215-0.
^ Berlyn GP, Miksche JP (1976). Botanical Microtechnique and Cytochemistry. Iowa State University Press.
^ Baker JR (1958). Principles of Biological Microtechnique. pp. 329 ff. London: Methuen.
^ Kiernan, J. A. (2001). "Classification and naming of dyes, stains and fluorochromes". Biotechnic & Histochemistry. 76 (5–6): 261–78. doi:10.1080/bih.76.5-6.261.278. PMID 11871748.
^ thefreedictionary.com > amphophilic Citing: Saunders Comprehensive Veterinary Dictionary, 3 ed. 2007 Elsevier, Inc
Further reading
- Bancroft JD, Gamble M eds (2002) Theory and Practice of Histological Techniques. 5th ed. London: Churchill-Livingstone.
ISBN 0443064350. - Kiernan JA (2015) Histological and Histochemical Methods. Theory and Practice. Banbury, UK: Scion.
ISBN 9781907904325. - Presnell JK, Schreibman MP (1997) Humason's Animal tissue Techniques. 5th ed. Baltimore: Johns Hopkins University Press.
- Ruzin SE (1999) Plant Microtechnique and Microscopy. New York: Oxford University Press.
ISBN 0195089561
External links
Library resources about Staining |
|
| Wikimedia Commons has media related to Staining. |
The Biological Stain commission is an independent non-profit company that has been testing dyes since the early 1920s and issuing Certificates of approval for batches of dyes that meet internationally recognized standards.
StainsFile Reference for dyes and staining techniques.
Vital Staining for Protozoa and Related Temporary Mounting Techniques ~ Howey, 2000
Speaking of Fixation: Part 1 and Part 2 – by M. Halit Umar- Photomicrographs of Histology Stains
Frequently asked questions in staining exercises at Sridhar Rao P.N's home page