Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R′ of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst.[1] The reaction can also be accomplished with the help of enzymes (biocatalysts) particularly lipases (E.C.3.1.1.3).
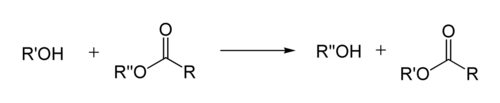
Transesterification: alcohol + ester → different alcohol + different ester
Strong acids catalyse the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile, whereas bases catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophilic. Esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol and evaporating the small alcohol to drive equilibrium.
Contents
1 Mechanism
2 Applications
2.1 Polyester production
2.2 Methanolysis and biodiesel production
2.3 High-pressure transesterification
3 See also
4 References
Mechanism
In the transesterification mechanism, the carbonyl carbon of the starting ester react to give a tetrahedral intermediate, which either reverts to the starting material, or proceeds to the transesterified product (RCOOR2). The various species exist in equilibrium, and the product distribution depends on the relative energies of the reactant and product.
Applications
Polyester production
The largest scale application of transesterification is in the synthesis of polyesters.[2] In this application diesters undergo transesterification with diols to form macromolecules. For example, dimethyl terephthalate and ethylene glycol react to form polyethylene terephthalate and methanol, which is evaporated to drive the reaction forward.
Methanolysis and biodiesel production
The reverse reaction, methanolysis, is also an example of transesterification. This process has been used to recycle polyesters into individual monomers (see plastic recycling). It is also used to convert fats (triglycerides) into biodiesel. This conversion was one of the first uses. Transesterified vegetable oil (biodiesel) was used to power heavy-duty vehicles in South Africa before World War II.
It was patented in the US in the 1950s by Colgate, though biolipid transesterification may have been discovered much earlier. In the 1940s, researchers were looking for a method to more readily produce glycerol, which was used to produce explosives for World War II. Many of the methods used today by producers have their origin in the original 1940s research.
Biolipid transesterification has also been recently shown by Japanese researchers to be possible using a super-critical methanol methodology, whereby high temperature, high-pressure vessels are used to physically catalyze the biolipid/methanol reaction into fatty-acid methyl esters.
High-pressure transesterification
Base-catalyzed transesterification is characterized by a negative activation volume (approx. −12 cm3/mol) and therefore it proceeds faster under high-pressure conditions. It has been shown that amine-catalyzed alcoholysis of sterically hindered esters (e.g. protecting groups, chiral auxiliaries) proceeds rapidly at room temperature under 10 kbar pressure, giving quantitative yields.[3]
See also
- Biodiesel production
- Interesterified fat
- Otera's catalyst
- Transalkylation
- Transamidification
- Cocaethylene
References
^ Otera, Junzo. (June 1993). "Transesterification". Chemical Reviews. 93 (4): 1449–1470. doi:10.1021/cr00020a004..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Wilhelm Riemenschneider1 and Hermann M. Bolt "Esters, Organic" Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a09_565.pub2
^ Romanski, J.; Nowak, P.; Kosinski, K.; Jurczak, J. (Sep 2012). "High-pressure transesterification of sterically hindered esters". Tetrahedron Lett. 53 (39): 5287–5289. doi:10.1016/j.tetlet.2012.07.094.
