Fluoxetine
 | |
 Fluoxetine (top), (R)-fluoxetine (center), (S)-fluoxetine (bottom) | |
| Clinical data | |
|---|---|
| Pronunciation | /fluˈɒksətiːn/ |
| Trade names | Prozac, Sarafem, Adofen, other |
AHFS/Drugs.com | Monograph |
| MedlinePlus | a689006 |
| License data |
|
| Pregnancy category |
|
| Dependence liability | Physical: Low Psychological: Low |
| Addiction liability | None |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
Pharmacokinetic data | |
| Bioavailability | 60–80%[2] |
| Protein binding | 94–95%[3] |
| Metabolism | Liver (mostly CYP2D6-mediated)[1] |
| Elimination half-life | 1–3 days (acute) 4–6 days (chronic)[1][4] |
| Excretion | Urine (80%), faeces (15%)[1][4] |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| ECHA InfoCard | 100.125.370 |
| Chemical and physical data | |
| Formula | C17H18F3NO |
| Molar mass | 309.33 g·mol−1 |
| 3D model (JSmol) |
|
| Chirality | Racemic mixture |
| Melting point | 179 to 182 °C (354 to 360 °F) |
| Boiling point | 395 °C (743 °F) |
| Solubility in water | 14 mg/mL (20 °C) |
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Fluoxetine, also known by trade names Prozac and Sarafem, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class.[2] It is used for the treatment of major depressive disorder, obsessive–compulsive disorder (OCD), bulimia nervosa, panic disorder and premenstrual dysphoric disorder.[2] It may decrease the risk of suicide in those over the age of 65.[2] It has also been used to treat premature ejaculation.[2] Fluoxetine is taken by mouth.[2]
Common side effects include trouble sleeping, sexual dysfunction, loss of appetite, dry mouth, rash and abnormal dreams.[2] Serious side effects include serotonin syndrome, mania, seizures, an increased risk of suicidal behavior in people under 25 years old and an increased risk of bleeding.[2] If stopped suddenly, a withdrawal syndrome may occur with anxiety, dizziness and changes in sensation.[2] It is unclear if it is safe in pregnancy.[5] If already on the medication, it may be reasonable to continue during breastfeeding.[5] Its mechanism of action is not entirely clear but believed to be related to increasing serotonin activity in the brain.[2]
Fluoxetine was discovered by Eli Lilly and Company in 1972 and entered medical use in 1986.[6] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[7] It is available as a generic medication.[2] The wholesale cost in the developing world is between US$0.01 and US$0.04 per day as of 2014.[8] In the United States, it costs about US$0.85 per day.[2] In 2016 it was the 29th most prescribed medication in the United States with more than 23 million prescriptions.[9]
Contents
1 Medical uses
1.1 Depression
1.2 Obsessive–compulsive disorder
1.3 Panic disorder
1.4 Bulimia nervosa
1.5 Premenstrual dysphoric disorder
1.6 Special populations
2 Adverse effects
2.1 Sexual dysfunction
2.2 Discontinuation syndrome
2.3 Suicide
3 Overdose
4 Interactions
5 Pharmacology
5.1 Pharmacodynamics
5.2 Pharmacokinetics
5.3 Measurement in body fluids
6 Usage
7 History
8 Society and culture
8.1 Airline pilots
8.2 Environmental effects
8.3 Politics
9 Research
9.1 Violence
10 See also
11 References
12 External links
Medical uses

Fluoxetine 20 mg blister pack

10 mg fluoxetine pills
Fluoxetine is frequently used to treat major depressive disorder, obsessive–compulsive disorder (OCD), post-traumatic stress disorder (PTSD), bulimia nervosa, panic disorder, premenstrual dysphoric disorder, and trichotillomania.[10][11][12][13] It has also been used for cataplexy, obesity, and alcohol dependence,[14] as well as binge eating disorder.[15] It has also been tried as a treatment for autism spectrum disorders with moderate success in adults.[16][17][18][19]
Depression
The effectiveness of fluoxetine and other antidepressants in the treatment of mild-to-moderate depression is controversial. A review of the comparative efficacy of 21 antidepressant drugs found that fluoxetine was among the least effective for treatment of depression.[20] A meta-analysis published by Kirsch in 2008 suggests, in those with mild or moderate symptoms, the efficacy of fluoxetine and other SSRIs is clinically insignificant.[21] A 2009 meta-analysis by Fournier which evaluated patient-level data from six trials of the SSRI paroxetine and the non-SSRI antidepressant imipramine has been further cited as evidence that antidepressants exhibit minimal efficacy in mild to moderate depression.[22] A 2012 meta-analysis using individual patient level-data of fluoxetine for the treatment of depression concluded statistically and clinically significant benefit was seen irrespective of baseline depression severity, and no significant effect was found on baseline severity on observed efficacy.[23] Overall there is no evidence from randomized controlled trials that fluoxetine or other SSRIs decrease the risk of suicide.[24] There is tentative evidence that suggests it may decrease the risk of suicide in those over the age of 65.[2]
A 2009 systematic review by the National Institute of Care and Clinical Excellence (NICE) (which considered the Kirsch, but not the later meta-analyses) concluded strong evidence existed for the efficacy of SSRIs in the treatment of moderate and severe depression, with some evidence for their efficacy in the treatment of mild depression.[25] Both the NICE and the Fournier analyses concluded that greater evidence is seen for the efficacy of antidepressants in the treatment of chronic mild depression (dysthymia) than in recent-onset mild depression.
NICE recommends antidepressant treatment with an SSRI in combination with psychosocial interventions as second-line treatment for short term mild depression, and as a first line treatment for severe and moderate depression, as well as mild depression that is recurrent or long-standing. The American Psychiatric Association includes antidepressant therapy among its first-line options for the treatment of depression, particularly when "a history of prior positive response to antidepressant medications, the presence of moderate to severe symptoms, significant sleep or appetite disturbances, agitation, patient preference, and anticipation of the need for maintenance therapy" exist.[26]
Obsessive–compulsive disorder
The efficacy of fluoxetine in the treatment of obsessive–compulsive disorder (OCD) was demonstrated in two randomized multicenter phase III clinical trials. The pooled results of these trials demonstrated that 47% of completers treated with the highest dose were "much improved" or "very much improved" after 13 weeks of treatment, compared to 11% in the placebo arm of the trial.[3] The American Academy of Child and Adolescent Psychiatry state that SSRIs, including fluoxetine, should be used as first-line therapy in children, along with cognitive behavioral therapy (CBT), for the treatment of moderate to severe OCD.[27]
Panic disorder
The efficacy of fluoxetine in the treatment of panic disorder was demonstrated in two 12-week randomized multicenter phase III clinical trials that enrolled patients diagnosed with panic disorder, with or without agoraphobia. In the first trial, 42% of subjects in the fluoxetine-treated arm were free of panic attacks at the end of the study, vs. 28% in the placebo arm. In the second trial, 62% of fluoxetine treated patients were free of panic attacks at the end of the study, vs. 44% in the placebo arm.[3]
Bulimia nervosa
A 2011 systematic review of seven trials which compared fluoxetine to a placebo in the treatment of bulimia nervosa; six of which found a statistically significant reduction in symptoms such as vomiting and binge eating.[28] However, no difference was observed between treatment arms when fluoxetine and psychotherapy were compared to psychotherapy alone.
Premenstrual dysphoric disorder
Fluoxetine is used to treat premenstrual dysphoric disorder.[29][30]
Special populations
In children and adolescents, fluoxetine is the antidepressant of choice due to tentative evidence favoring its efficacy and tolerability.[31][32] In pregnancy, fluoxetine is considered a category C drug by the USA FDA. Evidence supporting an increased risk of major fetal malformations resulting from fluoxetine exposure is limited, although the Medicines and Healthcare Products Regulatory Agency (MHRA) of the UK has warned prescribers and patients of the potential for fluoxetine exposure in the first trimester (during organogenesis, formation of the fetal organs) to cause a slight increase in the risk of congenital cardiac malformations in the newborn.[33][34][35] Furthermore, an association between fluoxetine use during the first trimester and an increased risk of minor fetal malformations was observed in one study.[34]
However, a systematic review and meta-analysis of 21 studies – published in the Journal of Obstetrics and Gynaecology Canada – concluded, "the apparent increased risk of fetal cardiac malformations associated with maternal use of fluoxetine has recently been shown also in depressed women who deferred SSRI therapy in pregnancy, and therefore most probably reflects an ascertainment bias. Overall, women who are treated with fluoxetine during the first trimester of pregnancy do not appear to have an increased risk of major fetal malformations."[36]
Per the FDA, infants exposed to SSRIs in late pregnancy may have an increased risk for persistent pulmonary hypertension of the newborn. Limited data support this risk, but the FDA recommends physicians consider tapering SSRIs such as fluoxetine during the third trimester.[3] A 2009 review recommended against fluoxetine as a first-line SSRI during lactation, stating, "Fluoxetine should be viewed as a less-preferred SSRI for breastfeeding mothers, particularly with newborn infants, and in those mothers who consumed fluoxetine during gestation."[37]Sertraline is often the preferred SSRI during pregnancy due to the relatively minimal fetal exposure observed and its safety profile while breastfeeding.[38]
Adverse effects
Side effects observed in fluoxetine-treated persons in clinical trials with an incidence >5% and at least twice as common in fluoxetine-treated persons compared to those who received a placebo pill include abnormal dreams, abnormal ejaculation, anorexia, anxiety, asthenia, diarrhea, dry mouth, dyspepsia, flu syndrome, impotence, insomnia, decreased libido, nausea, nervousness, pharyngitis, rash, sinusitis, somnolence, sweating, tremor, vasodilatation, and yawning.[39] Fluoxetine is considered the most stimulating of the SSRIs (that is, it is most prone to causing insomnia and agitation).[40] It also appears to be the most prone of the SSRIs for producing dermatologic reactions (e.g. urticaria (hives), rash, itchiness, etc.).[34]
Sexual dysfunction
Sexual dysfunction, including loss of libido, anorgasmia, lack of vaginal lubrication, and erectile dysfunction, are some of the most commonly encountered adverse effects of treatment with fluoxetine and other SSRIs. While early clinical trials suggested a relatively low rate of sexual dysfunction, more recent studies in which the investigator actively inquires about sexual problems suggest that the incidence is >70%.[41] Symptoms of sexual dysfunction have been reported to persist after discontinuing SSRIs, although this is thought to be occasional.[3][42][43]
Discontinuation syndrome
Antidepressant discontinuation syndrome is an adverse effect of second generation anti-depressants, including fluoxetine. The symptoms appear with rapid discontinuation of one of these drugs, and can include dizziness, disturbance of balance, headache, nausea, insomnia, and vivid dreams. Others can include sensations of tingling or numbness, ‘electric-shock’-like sensations, and irritability, with some case reports of hallucinations. They can generally be prevented by tapering off the drug over a period of four weeks, although evidence is weak for optimal tapering and there is disagreement between experts over the schedule. If a person is informed of the risk of discontinuation syndrome prior to starting the drug and again prior to beginning any tapering, discontinuation symptoms appear to be fewer and less severe, but again evidence is weak. Slower-acting drugs, like fluoxetine, may be less likely to cause discontinuation symptoms, but the evidence for this is weak as well. The mechanism by which discontinuation syndrome occurs in some people is not well understood.[44]
Suicide
In 2007 the FDA required all antidepressants to carry a black box warning stating that antidepressants may increase the risk of suicide in people younger than 25.[45] This warning is based on statistical analyses conducted by two independent groups of FDA experts that found a 2-fold increase of the suicidal ideation and behavior in children and adolescents, and 1.5-fold increase of suicidality in the 18–24 age group. The suicidality was slightly decreased for those older than 24, and statistically significantly lower in the 65 and older group.[46][47][48] This analysis was criticized by Donald Klein, who noted that suicidality, that is suicidal ideation and behavior, is not necessarily a good surrogate marker for completed suicide, and it is still possible that antidepressants may prevent actual suicide while increasing suicidality.[49]
There is less data on fluoxetine than on antidepressants as a whole. For the above analysis on the antidepressant level, the FDA had to combine the results of 295 trials of 11 antidepressants for psychiatric indications to obtain statistically significant results. Considered separately, fluoxetine use in children increased the odds of suicidality by 50%,[50] and in adults decreased the odds of suicidality by approximately 30%.[47][48] Similarly, the analysis conducted by the UK MHRA found a 50% increase of odds of suicide-related events, not reaching statistical significance, in the children and adolescents on fluoxetine as compared to the ones on placebo. According to the MHRA data, for adults fluoxetine did not change the rate of self-harm and statistically significantly decreased suicidal ideation by 50%.[51][52]
Overdose
In overdose, most frequent adverse effects include:[53]
Nervous system effects
| Gastrointestinal effects
| Other effects
|
Interactions
Contraindications include prior treatment (within the past two weeks) with MAOIs such as phenelzine and tranylcypromine, due to the potential for serotonin syndrome.[1] Its use should also be avoided in those with known hypersensitivities to fluoxetine or any of the other ingredients in the formulation used.[1] Its use in those concurrently receiving pimozide or thioridazine is also advised against.[1]
In some cases, use of dextromethorphan-containing cold and cough medications with fluoxetine is advised against, due to fluoxetine increasing serotonin levels, as well as the fact that fluoxetine is a cytochrome P450 2D6 inhibitor, which causes dextromethorphan to not be metabolized at a normal rate, thus increasing the risk of serotonin syndrome and other potential side effects of dextromethorphan.[54]
Patients who are taking anticoagulants or NSAIDS must be careful when taking fluoxetine or other SSRIs, as they can sometimes increase the blood-thinning effects of these medications.[55]
Fluoxetine and norfluoxetine inhibit many isozymes of the cytochrome P450 system that are involved in drug metabolism. Both are potent inhibitors of CYP2D6 (which is also the chief enzyme responsible for their metabolism) and CYP2C19, and mild to moderate inhibitors of CYP2B6 and CYP2C9.[56][57]In vivo, fluoxetine and norfluoxetine do not significantly affect the activity of CYP1A2 and CYP3A4.[56] They also inhibit the activity of P-glycoprotein, a type of membrane transport protein that plays an important role in drug transport and metabolism and hence P-glycoprotein substrates such as loperamide may have their central effects potentiated.[58] This extensive effect on the body's pathways for drug metabolism creates the potential for interactions with many commonly used drugs.[58][59]
Its use should also be avoided in those receiving other serotonergic drugs such as monoamine oxidase inhibitors, tricyclic antidepressants, methamphetamine, MDMA, triptans, buspirone, serotonin–norepinephrine reuptake inhibitors and other SSRIs due to the potential for serotonin syndrome to develop as a result.[1]
There is also the potential for interaction with highly protein-bound drugs due to the potential for fluoxetine to displace said drugs from the plasma or vice versa hence increasing serum concentrations of either fluoxetine or the offending agent.[1]
Pharmacology
| Molecular Target | Fluoxetine | Norfluoxetine |
|---|---|---|
| SERT | 1 | 19 |
| NET | 660 | 2700 |
| DAT | 4180 | 420 |
| 5-HT2A | 200 | 300 |
| 5-HT2B | 5000 | 5100 |
| 5-HT2C | 72.6 | 91.2 |
| α1 | 3000 | 3900 |
| M1 | 870 | 1200 |
| M2 | 2700 | 4600 |
| M3 | 1000 | 760 |
| M4 | 2900 | 2600 |
| M5 | 2700 | 2200 |
| H1 | 3250 | 10000 |
Entries with this color indicate a lower Ki bound. | ||
Pharmacodynamics
Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) and does not appreciably inhibit norepinephrine and dopamine reuptake at therapeutic doses. It does, however, delay the reuptake of serotonin, resulting in serotonin persisting longer when it is released. Large doses in rats have been shown to induce a significant increase in synaptic norepinephrine and dopamine.[62][63][64][65] Thus, dopamine and norepinephrine may contribute to the antidepressant action of fluoxetine in humans at supratherapeutic doses (60–80 mg).[64][66] This effect may be mediated by 5HT2C receptors, which are inhibited by higher concentrations of fluoxetine.[67]
Fluoxetine increases the concentration of circulating allopregnanolone, a potent GABAA receptor positive allosteric modulator, in the brain.[65][68]Norfluoxetine, a primary active metabolite of fluoxetine, produces a similar effect on allopregnanolone levels in the brains of mice.[65] Additionally, both fluoxetine and norfluoxetine are such modulators themselves, actions which may be clinically-relevant.[69]
In addition, fluoxetine has been found to act as an agonist of the σ1-receptor, with a potency greater than that of citalopram but less than that of fluvoxamine. However, the significance of this property is not fully clear.[70][71] Fluoxetine also functions as a channel blocker of anoctamin 1, a calcium-activated chloride channel.[72][73] A number of other ion channels, including nicotinic acetylcholine receptors and 5-HT3 receptors, are also known to be inhibited at similar concentrations.[69]
Fluoxetine has been shown to inhibit acid sphingomyelinase, a key regulator of ceramide levels which derives ceramide from sphingomyelin.[74][75]
Pharmacokinetics
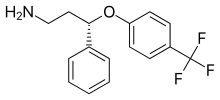
Seproxetine ((S)-norfluoxetine) – fluoxetine's chief active metabolite.
The bioavailability of fluoxetine is relatively high (72%), and peak plasma concentrations are reached in 6–8 hours. It is highly bound to plasma proteins, mostly albumin and α1-glycoprotein.[1] Fluoxetine is metabolized in the liver by isoenzymes of the cytochrome P450 system, including CYP2D6.[76] The role of CYP2D6 in the metabolism of fluoxetine may be clinically important, as there is great genetic variability in the function of this enzyme among people. CYP2D6 is responsible for converting fluoxetine to its only active metabolite, norfluoxetine.[77] Both drugs are also potent inhibitors of CYP2D6.[78]
The extremely slow elimination of fluoxetine and its active metabolite norfluoxetine from the body distinguishes it from other antidepressants. With time, fluoxetine and norfluoxetine inhibit their own metabolism, so fluoxetine elimination half-life changes from 1 to 3 days, after a single dose, to 4 to 6 days, after long-term use.[1] Similarly, the half-life of norfluoxetine is longer (16 days) after long-term use.[76][79][80] Therefore, the concentration of the drug and its active metabolite in the blood continues to grow through the first few weeks of treatment, and their steady concentration in the blood is achieved only after four weeks.[81][82] Moreover, the brain concentration of fluoxetine and its metabolites keeps increasing through at least the first five weeks of treatment.[83] That means that the full benefits of the current dose a patient receives are not realized for at least a month since its initiation. For example, in one 6-week study, the median time to achieving consistent response was 29 days.[81] Likewise, complete excretion of the drug may take several weeks. During the first week after the treatment discontinuation, the brain concentration of fluoxetine decreases only by 50%,[83] The blood level of norfluoxetine 4 weeks after the treatment discontinuation is about 80% of the level registered by the end of the first treatment week, and 7 weeks after the discontinuation norfluoxetine is still detectable in the blood.[79]
Measurement in body fluids
Fluoxetine and norfluoxetine may be quantitated in blood, plasma or serum to monitor therapy, confirm a diagnosis of poisoning in hospitalized patients or assist in a medicolegal death investigation. Blood or plasma fluoxetine concentrations are usually in a range of 50–500 μg/L in persons taking the drug for its antidepressant effects, 900–3000 μg/L in survivors of acute overdosage and 1000–7000 μg/L in victims of fatal overdosage. Norfluoxetine concentrations are approximately equal to those of the parent drug during chronic therapy, but may be substantially less following acute overdosage, since it requires at least 1–2 weeks for the metabolite to achieve equilibrium.[84][85][86]
Usage
In 2010, over 24.4 million prescriptions for generic fluoxetine were filled in the United States,[87] making it the third-most prescribed antidepressant after sertraline and citalopram.[87] In 2011, 6 million prescriptions for fluoxetine were filled in the United Kingdom.[88]
History
The work which eventually led to the discovery of fluoxetine began at Eli Lilly and Company in 1970 as a collaboration between Bryan Molloy and Robert Rathbun. It was known at that time that the antihistamine diphenhydramine shows some antidepressant-like properties. 3-Phenoxy-3-phenylpropylamine, a compound structurally similar to diphenhydramine, was taken as a starting point, and Molloy synthesized dozens of its derivatives.[89] Hoping to find a derivative inhibiting only serotonin reuptake, an Eli Lilly scientist, David T. Wong, proposed to retest the series for the in vitro reuptake of serotonin, norepinephrine and dopamine. This test, carried out by Jong-Sir Horng in May 1972,[89] showed the compound later named fluoxetine to be the most potent and selective inhibitor of serotonin reuptake of the series.[90] Wong published the first article about fluoxetine in 1974.[90] A year later, it was given the official chemical name fluoxetine and the Eli Lilly and Company gave it the trade name Prozac. In February 1977, Dista Products Company, a division of Eli Lilly & Company, filed an Investigational New Drug application to the U.S. Food and Drug Administration (FDA) for fluoxetine.[91]
Fluoxetine appeared on the Belgian market in 1986.[92] In the U.S., the FDA gave its final approval in December 1987,[93] and a month later Eli Lilly began marketing Prozac; annual sales in the U.S. reached $350 million within a year.[91] Worldwide sales eventually reached a peak of $2.6 billion a year.[94]
Lilly tried several product line extension strategies, including extended release formulations and paying for clinical trials to test the efficacy and safety of fluoxetine in premenstrual dysphoric disorder and rebranding the drug in that indication as "Sarafem" after it was approved by the FDA in 2000, following the recommendation of an advisory committee in 1999.[95][96][97] The invention of using fluoxetine to treat PMDD was made by Richard Wurtman at MIT, and the patent was licensed to his startup, Interneuron, which in turn sold it to Lilly.[98]
To defend its revenue from fluoxetine, Lilly also fought a five-year, multimillion-dollar battle in court with the generic company Barr Pharmaceuticals to protect its patents on fluoxetine, and lost the cases for its line-extension patents other than those for Sarafem, opening fluoxetine to generic manufacturers starting in 2001.[99] When Lilly's patent expired in August 2001,[100]generic drug competition decreased Lilly's sales of fluoxetine by 70% within two months.[95]
In 2000 an investment bank had projected that annual sales of Sarafem could reach $250M/year.[101] Sales of Sarafem reached about $85M/year in 2002, and in that year Lilly sold its assets around the drug for $295M to Galen Holdings, a small Irish pharmaceutical company specializing in dermatology and women's health that had a sales force tasked to gynecologists' offices; analysts found the deal sensible since the annual sales of Sarafem made a difference to Galen, but not to Lilly.[102][103]
Bringing Sarafem to market harmed Lilly's reputation in some quarters. The diagnostic category of PMDD was controversial since it was first proposed in 1987, and Lilly's role in retaining it in the appendix of the DSM-IV-TR, the discussions for which got underway in 1998, has been criticized.[101] Lilly was criticized for inventing a disease in order to make money,[101] and for not innovating but rather just seeking ways to continue making money from existing drugs.[104] It was also criticized by the FDA and groups concerned with women's health for marketing Sarafem too aggressively when it was first launched; the campaign included a television commercial featuring a harried woman at the grocery store who asks herself if she has PMDD.[105]
Society and culture
Airline pilots
Beginning April 5, 2010, fluoxetine became one of four antidepressant drugs that the FAA permitted for pilots with authorization from an aviation medical examiner. The other permitted antidepressants are sertraline (Zoloft), citalopram (Celexa), and escitalopram (Lexapro).[106] These four remain the only antidepressants permitted by FAA as of 2 December 2016.[ref][107]
Environmental effects
Fluoxetine has been detected in aquatic ecosystems, especially in North America.[108] There is a growing body of research addressing the effects of fluoxetine (among other SSRIs) exposure on non-target aquatic species.[109][110][111][112] In 2003, one of the first studies addressed in detail the potential effects of fluoxetine on aquatic wildlife; this research concluded that exposure at environmental concentrations was of little risk to aquatic systems if a hazard quotient approach was applied to risk assessment.[111] However, they also stated the need for further research addressing sub-lethal consequences of fluoxetine, specifically focusing on study species sensitivity, behavioural responses, and endpoints modulated by serotonin system.[111] Since this time, a number of studies have reported fluoxetine-induced impacts on a number of behavioural and physiological endpoints, inducing antipredator behaviour,[113][114][115] reproduction,[116][117][117] and foraging[118][119] at or below field-detected concentrations. However, a 2014 review on the ecotoxicology of fluoxetine concluded that at that time a consensus on the ability of environmental realistic dosages to affect the behaviour of wildlife could not be reached.[110]
Politics
During the 1990 campaign for Governor of Florida, it was disclosed that one of the candidates, Lawton Chiles, had depression and had resumed taking fluoxetine, leading his political opponents to question his fitness to serve as Governor.[120]
Research
Violence
Neither the American Psychiatric Association,[26] the National Institute for Health and Care Excellence (NICE),[121] nor the American College of Physicians[122] list violence among the potential side effects of treatment with serotonin selective reuptake inhibitors. Similarly, the World Health Organization and the European Psychiatric Association do not list violence among the potential side effects of SSRIs.[123][124]
Serial case report studies of this type have been criticized as being subject to "confounding by indication", in which effects due to an underlying disease state are mistakenly attributed to the effects of treatment.[125] Other studies, including randomized clinical trials and observational studies, have suggested that fluoxetine and other SSRIs may reduce the propensity for violence. A randomized clinical trial performed by the US National Institutes for Mental Health found that fluoxetine reduced acts of domestic violence in alcoholics with a history of such behavior[126] A second clinical trial performed at the University of Chicago found that fluoxetine reduced aggressive behavior in patients in intermittent aggressive disorder.[127] A clinical trial found that fluoxetine reduced aggressive behavior in patients with borderline personality disorder.[128] These results are indirectly supported by studies demonstrating that other SSRIs can reduce violence and aggressive behavior.[129][130][131][132] A NBER study examining international trends in antidepressant use and crime rates in the 1990s found that increases in antidepressant drug prescriptions were associated with reductions in violent crime.[133]
Despite the above cited evidence, psychiatrist David Healy and certain patient activist groups have compiled case reports of violent acts committed by individuals taking fluoxetine or other SSRIs,[134][135] and have argued that these drugs predispose susceptible individuals to commit violent acts.
See also
Atomoxetine—modified base and same termination of the molecule; it is a variant of the same structure
References
^ abcdefghij "PROZAC® Fluoxetine Hydrochloride" (PDF). TGA eBusiness Services. Eli Lilly Australia Pty. Limited. 9 October 2013. Archived from the original on 25 April 2017. Retrieved 23 November 2013..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcdefghijklm "Fluoxetine Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
^ abcde "Prozac Label" (PDF). FDA. 2014. Archived (PDF) from the original on 4 March 2016. Retrieved 5 April 2016.
^ ab Altamura AC, Moro AR, Percudani M (March 1994). "Clinical pharmacokinetics of fluoxetine" (PDF). Clinical Pharmacokinetics. 26 (3): 201–14. doi:10.2165/00003088-199426030-00004. PMID 8194283.
^ ab "Fluoxetine Pregnancy and Breastfeeding Warnings". Archived from the original on 8 September 2017. Retrieved 2 December 2015.
^ Myers RL (2007). The 100 most important chemical compounds: a reference guide (1st ed.). Westport, Conn.: Greenwood Press. p. 128. ISBN 978-0-313-33758-1.
^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
^ Frye JE, Johnson K, eds. (2014). "Fluoxetine". International Drug Price Indicator Guide (PDF). Medford, Massachusetts: Management Sciences for Health.
^ "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
^ Hagerman RJ (16 September 1999). Neurodevelopmental Disorders: Diagnosis and Treatment. Oxford University Press. ISBN 978-0-19-512314-2.Dech and Budow (1991) were among the first to report the anecdotal use of fluoxetine in a case of PWS to control behavior problems, appetite, and trichotillomania.
^ Truven Health Analytics, Inc. DrugPoint® System (Internet) [cited 2013 Oct 4]. Greenwood Village, CO: Thomsen Healthcare; 2013.
^ Australian Medicines Handbook 2013. The Australian Medicines Handbook Unit Trust; 2013.
^ British National Formulary (BNF) 65. Pharmaceutical Pr; 2013.
^ "Fluoxetine Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 11 April 2011. Retrieved 3 April 2011.
^ "NIMH•Eating Disorders". The National Institute of Mental Health. National Institute of Health. 2011. Archived from the original on 19 August 2011. Retrieved 25 November 2013.
^ Williams K, Brignell A, Randall M, Silove N, Hazell P (August 2013). "Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD)". The Cochrane Database of Systematic Reviews. 8 (8): CD004677. doi:10.1002/14651858.CD004677.pub3. PMID 23959778.
^ Myers SM (August 2007). "The status of pharmacotherapy for autism spectrum disorders". Expert Opinion on Pharmacotherapy. 8 (11): 1579–603. doi:10.1517/14656566.8.11.1579. PMID 17685878.
^ Doyle CA, McDougle CJ (August 2012). "Pharmacotherapy to control behavioral symptoms in children with autism". Expert Opinion on Pharmacotherapy. 13 (11): 1615–29. doi:10.1517/14656566.2012.674110. PMID 22550944.
^ Benvenuto A, Battan B, Porfirio MC, Curatolo P (February 2013). "Pharmacotherapy of autism spectrum disorders". Brain & Development. 35 (2): 119–27. doi:10.1016/j.braindev.2012.03.015. PMID 22541665.
^ Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JP, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JP, Geddes JR (April 2018). "Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis". Lancet. 391 (10128): 1357–1366. doi:10.1016/S0140-6736(17)32802-7. PMC 5889788. PMID 29477251.
^ Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (February 2008). "Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration". PLoS Medicine. PLoS Med. 5 (2): e45. doi:10.1371/journal.pmed.0050045. PMC 2253608. PMID 18303940.
^ Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J (January 2010). "Antidepressant drug effects and depression severity: a patient-level meta-analysis". JAMA. 303 (1): 47–53. doi:10.1001/jama.2009.1943. PMC 3712503. PMID 20051569.
^ Gibbons RD, Hur K, Brown CH, Davis JM, Mann JJ (June 2012). "Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine". Archives of General Psychiatry. 69 (6): 572–9. doi:10.1001/archgenpsychiatry.2011.2044. PMC 3371295. PMID 22393205.
^ Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, Iversen M, Banke MB, Petersen IJ, Klingenberg SL, Krogh J, Ebert SE, Timm A, Lindschou J, Gluud C (February 2017). "Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis". BMC Psychiatry. 17 (1): 58. doi:10.1186/s12888-016-1173-2. PMC 5299662. PMID 28178949.
^ "CG90 Depression in adults: full guidance" (PDF). National Institute for Health and Care Excellence. Archived from the original on 9 January 2014.
^ ab Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, Van Rhoads RS, et al. (Work Group on Major Depressive Disorder) (October 2010). Reus VI, DePaulo JR, Fawcett JA, Schneck CD, Silbersweig DA, eds. "Practice Guideline for the Treatment of Patients With Major Depressive Disorder" (PDF) (Third ed.). American Psychiatric Association.
^ "Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder". Journal of the American Academy of Child and Adolescent Psychiatry. 51 (1): 98–113. January 2012. doi:10.1016/j.jaac.2011.09.019. PMID 22176943.
^ Aigner M, Treasure J, Kaye W, Kasper S (September 2011). "World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of eating disorders" (PDF). The World Journal of Biological Psychiatry. World Federation of Societies of Biological Psychiatry. 12 (6): 400–43. doi:10.3109/15622975.2011.602720. ISSN 1814-1412. PMID 21961502. Archived (PDF) from the original on 1 August 2014.
^ Sarafem label Archived 8 May 2016 at the Wayback Machine. Last updated October 2014
^ Rapkin AJ, Lewis EI (November 2013). "Treatment of premenstrual dysphoric disorder". Women's Health. 9 (6): 537–56. doi:10.2217/whe.13.62. PMID 24161307.
^ Taurines R, Gerlach M, Warnke A, Thome J, Wewetzer C (September 2011). "Pharmacotherapy in depressed children and adolescents". The World Journal of Biological Psychiatry. 12 Suppl 1 (Suppl 1): 11–5. doi:10.3109/15622975.2011.600295. PMID 21905988.
^ Cohen D (2007). "Should the use of selective serotonin reuptake inhibitors in child and adolescent depression be banned?". Psychotherapy and Psychosomatics. 76 (1): 5–14. doi:10.1159/000096360. PMID 17170559.
^ Morrison JL, Riggs KW, Rurak DW (March 2005). "Fluoxetine during pregnancy: impact on fetal development". Reproduction, Fertility, and Development. 17 (6): 641–50. doi:10.1071/RD05030. PMID 16263070.
^ abc Brayfield, A, ed. (13 August 2013). Fluoxetine Hydrochloride. Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 24 November 2013.
(subscription required)
^ "Fluoxetine in pregnancy: slight risk of heart defects in unborn child" (PDF). MHRA. Medicines and Healthcare Products Regulatory Agency. 10 September 2011. Archived from the original (PDF) on 2 December 2013. Retrieved 23 November 2013.
^ Rowe T (June 2015). "Drugs in Pregnancy". Journal of Obstetrics and Gynaecology Canada. 37 (6): 489–92. doi:10.1016/S1701-2163(15)30222-X. PMID 26334601.
^ Kendall-Tackett K, Hale TW (May 2010). "The use of antidepressants in pregnant and breastfeeding women: a review of recent studies". Journal of Human Lactation : Official Journal of International Lactation Consultant Association. 26 (2): 187–95. doi:10.1177/0890334409342071. PMID 19652194.
^ Taylor D, Paton C, Shitij K (2012). The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. ISBN 978-0-470-97948-8.
^ Bland RD, Clarke TL, Harden LB (February 1976). "Rapid infusion of sodium bicarbonate and albumin into high-risk premature infants soon after birth: a controlled, prospective trial". American Journal of Obstetrics and Gynecology. 124 (3): 263–7. doi:10.1016/0002-9378(76)90154-x. PMID 2013.
^ Koda-Kimble MA, Alldredge BK (2012). Applied therapeutics: the clinical use of drugs (10th ed.). Baltimore: Wolters Kluwer Health/Lippincott Williams & Wilkins. ISBN 978-1609137137.
^ Clark MS, Jansen K, Bresnahan M (November 2013). "Clinical inquiry: How do antidepressants affect sexual function?". The Journal of Family Practice. 62 (11): 660–1. PMID 24288712.
^ Csoka AB, Csoka A, Bahrick A, Mehtonen OP (January 2008). "Persistent sexual dysfunction after discontinuation of selective serotonin reuptake inhibitors". The Journal of Sexual Medicine. 5 (1): 227–33. doi:10.1111/j.1743-6109.2007.00630.x. PMID 18173768.
^ Csoka AB, Shipko S (2006). "Persistent sexual side effects after SSRI discontinuation" (PDF). Psychotherapy and Psychosomatics. 75 (3): 187–8. doi:10.1159/000091777. PMID 16636635.
^ Wilson E, Lader M (December 2015). "A review of the management of antidepressant discontinuation symptoms". Therapeutic Advances in Psychopharmacology. 5 (6): 357–68. doi:10.1177/2045125315612334. PMC 4722507. PMID 26834969.
^ FDA. May 2, 2007. Antidepressant Use in Children, Adolescents, and Adults Archived 6 January 2016 at the Wayback Machine.
^ Levenson M, Holland C. "Antidepressants and Suicidality in Adults: Statistical Evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006)". Archived from the original on 27 September 2007. Retrieved 13 May 2007.
^ ab Stone MB, Jones ML (17 November 2006). "Clinical Review: Relationship Between Antidepressant Drugs and Suicidality in Adults" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 11–74. Archived (PDF) from the original on 16 March 2007. Retrieved 22 September 2007.
^ ab Levenson M, Holland C (17 November 2006). "Statistical Evaluation of Suicidality in Adults Treated with Antidepressants" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 75–140. Archived (PDF) from the original on 16 March 2007. Retrieved 22 September 2007.
^ Klein DF (April 2006). "The flawed basis for FDA post-marketing safety decisions: the example of anti-depressants and children". Neuropsychopharmacology. 31 (4): 689–99. doi:10.1038/sj.npp.1300996. PMID 16395296.
^ Hammad TA (13 September 2004). "Results of the Analysis of Suicidality in Pediatric Trials of Newer Antidepressants" (PDF). Presentation at the Meeting of Psychopharmacologic Drugs Advisory Committee and the Pediatric Advisory Committee on September 13, 2004. FDA. Archived from the original on 28 February 2008.Pages 25, 28. Retrieved 2008-01-06.
^ Committee on Safety of Medicines Expert Working Group (December 2004). "Report on The Safety of Selective Serotonin Reuptake Inhibitor Antidepressants" (PDF). MHRA. Archived (PDF) from the original on 28 February 2008. Retrieved 25 September 2007.
^ Gunnell D, Saperia J, Ashby D (February 2005). "Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA's safety review". BMJ. 330 (7488): 385. doi:10.1136/bmj.330.7488.385. PMC 549105. PMID 15718537.
^ "Toxicity". Fluoxetine. PubChem. NCBI. Retrieved 13 March 2015.
^ "Dextromethorphan and fluoxetine Drug Interactions". Drugs.com. Archived from the original on 14 August 2017. Retrieved 3 March 2017.
^ "Fluoxetine and ibuprofen Drug Interactions". Drugs.com. Archived from the original on 31 August 2017. Retrieved 3 March 2017.
^ ab Sager JE, Lutz JD, Foti RS, Davis C, Kunze KL, Isoherranen N (June 2014). "Fluoxetine- and norfluoxetine-mediated complex drug-drug interactions: in vitro to in vivo correlation of effects on CYP2D6, CYP2C19, and CYP3A4". Clinical Pharmacology and Therapeutics. 95 (6): 653–62. doi:10.1038/clpt.2014.50. PMC 4029899. PMID 24569517.
^ Ciraulo DA, Shader RI, eds. (2011). Pharmacotherapy of Depression. SpringerLink (2nd ed.). New York, NY: Humana Press. doi:10.1007/978-1-60327-435-7. ISBN 978-1-60327-434-0. Archived from the original on 2013-11-15.
^ ab Sandson NB, Armstrong SC, Cozza KL (2005). "An overview of psychotropic drug-drug interactions". Psychosomatics. 46 (5): 464–94. doi:10.1176/appi.psy.46.5.464. PMID 16145193.
^ An extensive list of possible interactions is available in Lexi-Comp (September 2008). "Fluoxetine". The Merck Manual Professional. Archived from the original on 3 September 2007. Retrieved on December 28, 2008.
^ Roth BL, Driscol J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 November 2013. Retrieved 24 June 2013.
^ Owens MJ, Knight DL, Nemeroff CB (September 2001). "Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine". Biological Psychiatry. 50 (5): 345–50. doi:10.1016/s0006-3223(01)01145-3. PMID 11543737.
^ Perry KW, Fuller RW (1997). "Fluoxetine increases norepinephrine release in rat hypothalamus as measured by tissue levels of MHPG-SO4 and microdialysis in conscious rats". Journal of Neural Transmission. 104 (8–9): 953–66. doi:10.1007/BF01285563. PMID 9451727.
^ Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW (April 2002). "Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex". Psychopharmacology. 160 (4): 353–61. doi:10.1007/s00213-001-0986-x. PMID 11919662.
^ ab Koch S, Perry KW, Nelson DL, Conway RG, Threlkeld PG, Bymaster FP (December 2002). "R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: an in vivo microdialysis and receptor binding study". Neuropsychopharmacology. 27 (6): 949–59. doi:10.1016/S0893-133X(02)00377-9. PMID 12464452.
^ abc Pinna G, Costa E, Guidotti A (February 2009). "SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake". Current Opinion in Pharmacology. 9 (1): 24–30. doi:10.1016/j.coph.2008.12.006. PMC 2670606. PMID 19157982.
^ Miguelez C, Fernandez-Aedo I, Torrecilla M, Grandoso L, Ugedo L (2009). "alpha(2)-Adrenoceptors mediate the acute inhibitory effect of fluoxetine on locus coeruleus noradrenergic neurons". Neuropharmacology. 56 (6–7): 1068–73. doi:10.1016/j.neuropharm.2009.03.004. PMID 19298831.
^ Pälvimäki EP, Roth BL, Majasuo H, Laakso A, Kuoppamäki M, Syvälahti E, Hietala J (August 1996). "Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor". Psychopharmacology. 126 (3): 234–40. doi:10.1007/BF02246453. PMID 8876023.
^ Brunton PJ (June 2016). "Neuroactive steroids and stress axis regulation: Pregnancy and beyond". The Journal of Steroid Biochemistry and Molecular Biology. 160: 160–8. doi:10.1016/j.jsbmb.2015.08.003. PMID 26259885.
^ ab Robinson RT, Drafts BC, Fisher JL (March 2003). "Fluoxetine increases GABA(A) receptor activity through a novel modulatory site". The Journal of Pharmacology and Experimental Therapeutics. 304 (3): 978–84. doi:10.1124/jpet.102.044834. PMID 12604672.
^ Narita N, Hashimoto K, Tomitaka S, Minabe Y (June 1996). "Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain". European Journal of Pharmacology. 307 (1): 117–9. doi:10.1016/0014-2999(96)00254-3. PMID 8831113.
^ Hashimoto K (September 2009). "Sigma-1 receptors and selective serotonin reuptake inhibitors: clinical implications of their relationship". Central Nervous System Agents in Medicinal Chemistry. 9 (3): 197–204. doi:10.2174/1871524910909030197. PMID 20021354.
^ "Fluoxetine". IUPHAR Guide to Pharmacology. IUPHAR. Archived from the original on 10 November 2014. Retrieved 10 November 2014.
^ "Calcium activated chloride channel". IUPHAR Guide to Pharmacology. IUPHAR. Archived from the original on 10 November 2014. Retrieved 10 November 2014.
^ Gulbins E, Palmada M, Reichel M, Lüth A, Böhmer C, Amato D, et al. (July 2013). "Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs". Nature Medicine. 19 (7): 934–8. doi:10.1038/nm.3214. PMID 23770692.
^ Brunkhorst R, Friedlaender F, Ferreirós N, Schwalm S, Koch A, Grammatikos G, Toennes S, Foerch C, Pfeilschifter J, Pfeilschifter W (October 2015). "Alterations of the Ceramide Metabolism in the Peri-Infarct Cortex Are Independent of the Sphingomyelinase Pathway and Not Influenced by the Acid Sphingomyelinase Inhibitor Fluoxetine". Neural Plasticity. 2015: 503079. doi:10.1155/2015/503079. PMC 4641186. PMID 26605090.
^ ab "Prozac Pharmacology, Pharmacokinetics, Studies, Metabolism". RxList.com. 2007. Archived from the original on 10 April 2007. Retrieved 14 April 2007.
^ Mandrioli R, Forti GC, Raggi MA (February 2006). "Fluoxetine metabolism and pharmacological interactions: the role of cytochrome p450". Current Drug Metabolism. 7 (2): 127–33. doi:10.2174/138920006775541561. PMID 16472103.
^ Hiemke C, Härtter S (January 2000). "Pharmacokinetics of selective serotonin reuptake inhibitors". Pharmacology & Therapeutics. 85 (1): 11–28. doi:10.1016/S0163-7258(99)00048-0. PMID 10674711.
^ ab Burke WJ, Hendricks SE, McArthur-Miller D, Jacques D, Bessette D, McKillup T, Stull T, Wilson J (August 2000). "Weekly dosing of fluoxetine for the continuation phase of treatment of major depression: results of a placebo-controlled, randomized clinical trial". Journal of Clinical Psychopharmacology. 20 (4): 423–7. doi:10.1097/00004714-200008000-00006. PMID 10917403.
^ "Drug Treatments in Psychiatry: Antidepressants". Newcastle University School of Neurology, Neurobiology and Psychiatry. 2005. Archived from the original on 17 April 2007. Retrieved 14 April 2007.
^ ab Pérez V, Puiigdemont D, Gilaberte I, Alvarez E, Artigas F, et al. (Grup de Recerca en Trastorns Afectius) (February 2001). "Augmentation of fluoxetine's antidepressant action by pindolol: analysis of clinical, pharmacokinetic, and methodologic factors". Journal of Clinical Psychopharmacology. 21 (1): 36–45. doi:10.1097/00004714-200102000-00008. PMID 11199945.
^ Brunswick DJ, Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Beasley CM (April 2002). "Fluoxetine and norfluoxetine plasma concentrations during relapse-prevention treatment". Journal of Affective Disorders. 68 (2–3): 243–9. doi:10.1016/S0165-0327(00)00333-5. PMID 12063152.
^ ab Henry ME, Schmidt ME, Hennen J, Villafuerte RA, Butman ML, Tran P, Kerner LT, Cohen B, Renshaw PF (August 2005). "A comparison of brain and serum pharmacokinetics of R-fluoxetine and racemic fluoxetine: A 19-F MRS study". Neuropsychopharmacology. 30 (8): 1576–83. doi:10.1038/sj.npp.1300749. PMID 15886723.
^ Lemberger L, Bergstrom RF, Wolen RL, Farid NA, Enas GG, Aronoff GR (March 1985). "Fluoxetine: clinical pharmacology and physiologic disposition". The Journal of Clinical Psychiatry. 46 (3 Pt 2): 14–9. PMID 3871765.
^ Pato MT, Murphy DL, DeVane CL (June 1991). "Sustained plasma concentrations of fluoxetine and/or norfluoxetine four and eight weeks after fluoxetine discontinuation". Journal of Clinical Psychopharmacology. 11 (3): 224–5. doi:10.1097/00004714-199106000-00024. PMID 1741813.
^ Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 645–648.
^ ab "Top 200 Generic Drugs by Units in 2010" (PDF). Drug Topics: Voice of the Pharmacist. June 2011. Archived from the original (PDF) on 15 December 2012.
^ Macnair P (September 2012). "BBC – Health: Prozac". BBC. Archived from the original on 2012-12-11.In 2011 over 43 million prescriptions for antidepressants were handed out in the UK and about 14 per cent (or nearly 6 million prescriptions) of these were for a drug called fluoxetine, better known as Prozac.
^ ab Wong DT, Bymaster FP, Engleman EA (1995). "Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication". Life Sciences. 57 (5): 411–41. doi:10.1016/0024-3205(95)00209-O. PMID 7623609.
^ ab Wong DT, Horng JS, Bymaster FP, Hauser KL, Molloy BB (August 1974). "A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine". Life Sciences. 15 (3): 471–9. doi:10.1016/0024-3205(74)90345-2. PMID 4549929.
^ ab Breggin PR, Breggin GR (1995). Talking Back to Prozac. Macmillan Publishers. pp. 1–2. ISBN 978-0-312-95606-6.
^ Swiatek J (2 August 2001). "Prozac's profitable run coming to an end for Lilly". The Indianapolis Star. Archived from the original on 18 August 2007.
^ "Electronic Orange Book". Food and Drug Administration. April 2007. Archived from the original on 20 August 2007. Retrieved 24 May 2007.
^ Simons J (28 June 2004). "Lilly Goes Off Prozac The drugmaker bounced back from the loss of its blockbuster, but the recovery had costs". Fortune Magazine.
^ ab Class S (December 2, 2002). "Pharma Overview". Retrieved June 15, 2009.
^ "Lilly Menstrual drug OK'd – Jul. 6, 2000". Money.cnn.com. 6 July 2000. Archived from the original on 5 May 2016. Retrieved 3 March 2017.
^ Mechatie E (December 1, 1999). "FDA Panel Agrees Fluoxetine Effective For PMDD". International Medical News Group.
^ Herper M (25 September 2002). "A Biotech Phoenix Could Be Rising". Forbes.
^ Petersen M (2 August 2001). "Drug Maker Is Set to Ship Generic Prozac". The New York Times.
^ "Patent Expiration Dates for Common Brand-Name Drugs". Archived from the original on 28 September 2007. Retrieved 20 July 2007.
^ abc Spartos C (2000-12-05). "Sarafem Nation". Village Voice. Retrieved 2017-03-03.
^ "Galen to Pay $295 Million For U.S. Rights to Lilly Drug". Dow Jones Newswires in the Wall Street Journal. 9 December 2002.
^ Murray-West R (10 December 2002). "Galen takes Lilly's reinvented Prozac". Telegraph.
^ Petersen M (29 May 2002). "New Medicines Seldom Contain Anything New, Study Finds". The New York Times.
^ Vedantam S (29 April 2001). "Renamed Prozac Fuels Women's Health Debate". The Washington Post.
^ Duquette A, Dorr L (2 April 2010). "FAA Proposes New Policy on Antidepressants for Pilots" (Press release). Washington, DC: Federal Aviation Administration, U.S. Department of Transportation. Archived from the original on 14 January 2012. Retrieved 10 February 2012.
^ Office of Aerospace Medicine; Federal Aviation Administration (2 December 2016). "Decision Considerations – Aerospace Medical Dispositions: Item 47. Psychiatric Conditions – Use of Antidepressant Medications". Guide for Aviation Medical Examiners. Washington, DC: United States Department of Transportation. Archived from the original on 3 May 2017.
^ Hughes SR, Kay P, Brown LE (January 2013). "Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems". Environmental Science & Technology. 47 (2): 661–77. doi:10.1021/es3030148. PMC 3636779. PMID 23227929.
^ Stewart AM, Grossman L, Nguyen M, Maximino C, Rosemberg DB, Echevarria DJ, Kalueff AV (November 2014). "Aquatic toxicology of fluoxetine: understanding the knowns and the unknowns". Aquatic Toxicology. 156: 269–73. doi:10.1016/j.aquatox.2014.08.014. PMID 25245382.
^ ab Sumpter JP, Donnachie RL, Johnson AC (June 2014). "The apparently very variable potency of the anti-depressant fluoxetine". Aquatic Toxicology. 151: 57–60. doi:10.1016/j.aquatox.2013.12.010. PMID 24411166.
^ abc Brooks BW, Foran CM, Richards SM, Weston J, Turner PK, Stanley JK, Solomon KR, Slattery M, La Point TW (May 2003). "Aquatic ecotoxicology of fluoxetine". Toxicology Letters. Hot Spot Pollutants: Pharmaceuticals in the Environment. 142 (3): 169–83. doi:10.1016/S0378-4274(03)00066-3. PMID 12691711.
^ Mennigen JA, Stroud P, Zamora JM, Moon TW, Trudeau VL (2011-07-01). "Pharmaceuticals as neuroendocrine disruptors: lessons learned from fish on Prozac". Journal of Toxicology and Environmental Health. Part B, Critical Reviews. 14 (5–7): 387–412. doi:10.1080/10937404.2011.578559. PMID 21790318.
^ Martin JM, Saaristo M, Bertram MG, Lewis PJ, Coggan TL, Clarke BO, Wong BB (March 2017). "The psychoactive pollutant fluoxetine compromises antipredator behaviour in fish". Environmental Pollution. 222: 592–599. doi:10.1016/j.envpol.2016.10.010. PMID 28063712.
^ Barry MJ (2014-04-21). "Fluoxetine inhibits predator avoidance behavior in tadpoles". Toxicological & Environmental Chemistry. 96 (4): 641–649. doi:10.1080/02772248.2014.966713.
^ Painter MM, Buerkley MA, Julius ML, Vajda AM, Norris DO, Barber LB, Furlong ET, Schultz MM, Schoenfuss HL (December 2009). "Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas)". Environmental Toxicology and Chemistry. 28 (12): 2677–84. doi:10.1897/08-556.1. PMID 19405782.
^ Mennigen JA, Lado WE, Zamora JM, Duarte-Guterman P, Langlois VS, Metcalfe CD, Chang JP, Moon TW, Trudeau VL (November 2010). "Waterborne fluoxetine disrupts the reproductive axis in sexually mature male goldfish, Carassius auratus". Aquatic Toxicology. 100 (4): 354–64. doi:10.1016/j.aquatox.2010.08.016. PMID 20864192.
^ ab Schultz MM, Painter MM, Bartell SE, Logue A, Furlong ET, Werner SL, Schoenfuss HL (July 2011). "Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows". Aquatic Toxicology. 104 (1–2): 38–47. doi:10.1016/j.aquatox.2011.03.011. PMID 21536011.
^ Mennigen JA, Sassine J, Trudeau VL, Moon TW (October 2010). "Waterborne fluoxetine disrupts feeding and energy metabolism in the goldfish Carassius auratus". Aquatic Toxicology. 100 (1): 128–37. doi:10.1016/j.aquatox.2010.07.022. PMID 20692053.
^ Gaworecki KM, Klaine SJ (July 2008). "Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure". Aquatic Toxicology. 88 (4): 207–13. doi:10.1016/j.aquatox.2008.04.011. PMID 18547660.
^ MacPherson M (2 September 1990). "Prozac, Prejudice and the Politics Of Depression". The Washington Post.
^ "Depression in adults: recognition and management – Guidance and guidelines". The National Institute for Health and Care Excellence (NICE) UK. Archived from the original on 29 June 2014. Retrieved 3 March 2017.
^ Gartlehner G, Gaynes BN, Hansen RA, Thieda P, DeVeaugh-Geiss A, Krebs EE, Moore CG, Morgan L, Lohr KN (November 2008). "Comparative benefits and harms of second-generation antidepressants: background paper for the American College of Physicians". Annals of Internal Medicine. 149 (10): 734–50. doi:10.7326/0003-4819-149-10-200811180-00008. PMID 19017592.
^ World Health Organization (2009). Pharmacological treatment of mental disorders in primary health care (PDF). Geneva, Switzerland: WHO Press.
^ Möller HJ, Bitter I, Bobes J, Fountoulakis K, Höschl C, Kasper S (February 2012). "Position statement of the European Psychiatric Association (EPA) on the value of antidepressants in the treatment of unipolar depression". European Psychiatry. 27 (2): 114–28. doi:10.1016/j.eurpsy.2011.08.002. PMID 22119161.
^ Shapiro S (July 2008). "Causation, bias and confounding: a hitchhiker's guide to the epidemiological galaxy Part 2. Principles of causality in epidemiological research: confounding, effect modification and strength of association". The Journal of Family Planning and Reproductive Health Care. 34 (3): 185–90. doi:10.1783/147118908784734873. PMID 18577320.
^ George DT, Phillips MJ, Lifshitz M, Lionetti TA, Spero DE, Ghassemzedeh N, Doty L, Umhau JC, Rawlings RR (January 2011). "Fluoxetine treatment of alcoholic perpetrators of domestic violence: a 12-week, double-blind, randomized, placebo-controlled intervention study". The Journal of Clinical Psychiatry. 72 (1): 60–5. doi:10.4088/JCP.09m05256gry. PMC 3026856. PMID 20673556.
^ Coccaro EF, Lee RJ, Kavoussi RJ (April 2009). "A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder". The Journal of Clinical Psychiatry. 70 (5): 653–62. doi:10.4088/JCP.08m04150. PMID 19389333.
^ Coccaro EF, Kavoussi RJ (December 1997). "Fluoxetine and impulsive aggressive behavior in personality-disordered subjects". Archives of General Psychiatry. 54 (12): 1081–8. doi:10.1001/archpsyc.1997.01830240035005. PMID 9400343.
^ Stark LJ, Spirito A, Williams CA, Guevremont DC (April 1989). "Common problems and coping strategies. I: Findings with normal adolescents". Journal of Abnormal Child Psychology. 17 (2): 203–12. doi:10.1007/BF00913794. PMID 2745900.
^ Berman ME, McCloskey MS, Fanning JR, Schumacher JA, Coccaro EF (June 2009). "Serotonin augmentation reduces response to attack in aggressive individuals". Psychological Science. 20 (6): 714–20. doi:10.1111/j.1467-9280.2009.02355.x. PMC 2728471. PMID 19422623.
^ McCloskey MS, Berman ME, Echevarria DJ, Coccaro EF (April 2009). "Effects of acute alcohol intoxication and paroxetine on aggression in men". Alcoholism, Clinical and Experimental Research. 33 (4): 581–90. doi:10.1111/j.1530-0277.2008.00872.x. PMID 19183141.
^ Cherek DR, Lane SD, Pietras CJ, Steinberg JL (January 2002). "Effects of chronic paroxetine administration on measures of aggressive and impulsive responses of adult males with a history of conduct disorder". Psychopharmacology. 159 (3): 266–74. doi:10.1007/s002130100915. PMID 11862359.
^ Marcotte DE, Markowitz S (September 2009). "A cure for crime? Psycho-pharmaceuticals and crime trends" (PDF). Journal of Policy Analysis and Management. Nber Working Paper Series. The National Bureau of Economic Research. 30 (1): 29–56. PMID 21465827. Archived (PDF) from the original on 2 December 2013. Retrieved 25 November 2013.
^ Healy D, Herxheimer A, Menkes DB (September 2006). "Antidepressants and violence: problems at the interface of medicine and law". PLoS Medicine. 3 (9): e372. doi:10.1371/journal.pmed.0030372. PMC 1564177. PMID 16968128.
^ Breggin PR, Breggin GR (1995). Talking Back to Prozac. Macmillan Publishers. p. 154. ISBN 978-0-312-95606-6.
External links
| Wikimedia Commons has media related to Fluoxetine. |
Fluoxetine, from the United States National Library of Medicine's Drug Information Portal
Shorter E (2014). "The 25th anniversary of the launch of Prozac gives pause for thought: where did we go wrong?". The British Journal of Psychiatry. 204: 331–2. doi:10.1192/bjp.bp.113.129916. PMID 24785765.
Prozac: Revolution in a Capsule - Retro Report - The New York Times on YouTube