Flavones

Molecular structure of the flavone backbone with numbers
Flavones (flavus = yellow), are a class of flavonoids based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one) (right image).[1][2]
Flavones are common in the food supply, mainly from spices, and red–purple fruits and vegetables.[1] Common flavones include apigenin (4',5,7-trihydroxyflavone), luteolin (3',4',5,7-tetrahydroxyflavone), tangeritin (4',5,6,7,8-pentamethoxyflavone), chrysin (5,7-dihydroxyflavone), and 6-hydroxyflavone.[1]
Contents
1 Intake and elimination
2 Drug interactions
3 Organic chemistry
3.1 Wessely–Moser rearrangement
4 Common flavones
5 References
6 External links
Intake and elimination
Flavones are mainly found in spices and red or purple plant foods.[1] The estimated daily intake of flavones is about 2 mg per day.[1] Flavones have no proven physiological effects in the human body and no antioxidant food value.[1][3] Following ingestion and metabolism, flavones, other polyphenols, and their metabolites are absorbed poorly in body organs and are rapidly excreted in the urine, indicating mechanisms influencing their presumed absence of metabolic roles in the body.[1][4]
Drug interactions
Flavones have effects on CYP (P450) activity [5][6] which are enzymes that metabolize most drugs in the body.
Organic chemistry
In organic chemistry several methods exist for the synthesis of flavones:
- Allan–Robinson reaction
- Auwers synthesis
- Baker–Venkataraman rearrangement
- Algar–Flynn–Oyamada reaction
Another method is the dehydrative cyclization of certain 1,3-diaryl diketones.[7]

Wessely–Moser rearrangement
The Wessely–Moser rearrangement (1930)[8] has been an important tool in structure elucidation of flavonoids. It involves the conversion of 5,7,8-trimethoxyflavone into 5,6,7-trihydroxyflavone on hydrolysis of the methoxy groups to phenol groups. It also has synthetic potential for example:[9]
This rearrangement reaction takes place in several steps: A ring opening to the diketone, B bond rotation with formation of a favorable acetylacetone-like phenyl-ketone interaction and C hydrolysis of two methoxy groups and ring closure.
Common flavones
| Name | Structure | R3 | R5 | R6 | R7 | R8 | R2' | R3' | R4' | R5' | R6' |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flavone backbone | 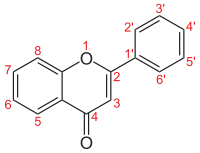 | – | – | – | – | – | – | – | – | – | – |
| Primuletin | – | –OH | – | – | – | – | – | – | – | – | |
Chrysin | – | –OH | – | –OH | – | – | – | – | – | – | |
Tectochrysin | – | –OH | – | –OCH3 | – | – | – | – | – | – | |
| Primetin | – | –OH | – | – | –OH | – | – | – | – | – | |
Apigenin | – | –OH | – | –OH | – | – | – | –OH | – | – | |
Acacetin | – | –OH | – | –OH | – | – | – | –OCH3 | – | – | |
Genkwanin | – | –OH | – | –OCH3 | – | – | – | –OH | – | – | |
| Echioidinin | – | –OH | – | –OCH3 | – | –OH | – | – | – | – | |
Baicalein | – | –OH | –OH | –OH | – | – | – | – | – | – | |
| Oroxylon | – | –OH | –OCH3 | –OH | – | – | – | – | – | – | |
| Negletein | – | –OH | –OH | –OCH3 | – | – | – | – | – | – | |
Norwogonin | – | –OH | – | –OH | –OH | – | – | – | – | – | |
Wogonin | – | –OH | – | –OH | –OCH3 | – | – | – | – | – | |
| Geraldone | – | – | – | –OH | – | – | –OCH3 | –OH | – | – | |
| Tithonine | – | – | – | –OCH3 | – | – | –OH | –OCH3 | – | – | |
Luteolin | – | –OH | – | –OH | – | – | –OH | –OH | – | – | |
6-Hydroxyluteolin | – | –OH | –OH | –OH | – | – | –OH | –OH | – | – | |
Chrysoeriol | – | –OH | – | –OH | – | – | –OCH3 | –OH | – | – | |
Diosmetin | – | –OH | – | –OH | – | – | –OH | –OCH3 | – | – | |
| Pilloin | – | –OH | – | –OCH3 | – | – | –OH | –OCH3 | – | – | |
| Velutin | – | –OH | – | –OCH3 | – | – | –OCH3 | –OH | – | – | |
Norartocarpetin | – | –OH | – | –OH | – | –OH | – | –OH | – | – | |
| Artocarpetin | – | –OH | – | –OCH3 | – | –OH | – | –OH | – | – | |
Scutellarein | – | –OH | –OH | –OH | – | – | – | –OH | – | – | |
Hispidulin | – | –OH | –OCH3 | –OH | – | – | – | –OH | – | – | |
| Sorbifolin | – | –OH | –OH | –OCH3 | – | – | – | –OH | – | – | |
Pectolinarigenin | – | –OH | –OCH3 | –OH | – | – | – | –OCH3 | – | – | |
| Cirsimaritin | – | –OH | –OCH3 | –OCH3 | – | – | – | –OH | – | – | |
| Mikanin | – | –OH | –OCH3 | –OCH3 | – | – | – | –OCH3 | – | – | |
Isoscutellarein | – | –OH | – | –OH | –OH | – | – | –OH | – | – | |
| Zapotinin | – | –OH | –OCH3 | – | – | –OCH3 | – | – | – | –OCH3 | |
Zapotin | – | –OCH3 | –OCH3 | – | – | –OCH3 | – | – | – | –OCH3 | |
| Cerrosillin | – | –OCH3 | –OCH3 | – | – | – | –OCH3 | – | –OCH3 | – | |
| Alnetin | – | –OH | –OCH3 | –OCH3 | –OCH3 | – | – | – | – | – | |
Tricetin | – | –OH | – | –OH | – | – | –OH | –OH | –OH | – | |
| Tricin | – | –OH | – | –OH | – | – | –OCH3 | –OH | –OCH3 | – | |
| Corymbosin | – | –OH | – | –OCH3 | – | – | –OCH3 | –OCH3 | –OCH3 | – | |
Nepetin | – | –OH | –OCH3 | –OH | – | – | –OH | –OH | – | – | |
| Pedalitin | – | –OH | –OH | –OCH3 | – | – | –OH | –OH | – | – | |
| Nodifloretin | – | –OH | –OH | –OH | – | – | –OCH3 | –OH | – | – | |
| Jaceosidin | – | –OH | –OCH3 | –OH | – | – | –OCH3 | –OH | – | – | |
| Cirsiliol | – | –OH | –OCH3 | –OCH3 | – | – | –OH | –OH | – | – | |
Eupatilin | – | –OH | –OCH3 | –OH | – | – | –OCH3 | –OCH3 | – | – | |
Cirsilineol | – | –OH | –OCH3 | –OCH3 | – | – | –OCH3 | –OH | – | – | |
| Eupatorin | – | –OH | –OCH3 | –OCH3 | – | – | – | –OCH3 | –OH | – | |
Sinensetin | – | –OCH3 | –OCH3 | –OCH3 | – | – | – | –OCH3 | –OCH3 | – | |
Hypolaetin | – | –OH | – | –OH | –OH | – | –OH | –OH | – | – | |
| Onopordin | – | –OH | – | –OH | –OCH3 | – | –OH | –OH | – | – | |
| Wightin | – | –OH | – | –OCH3 | –OCH3 | –OCH3 | –OH | – | – | – | |
| Nevadensin | – | –OH | –OCH3 | –OH | –OCH3 | – | – | –OCH3 | – | – | |
| Xanthomicrol | – | –OH | –OCH3 | –OCH3 | –OCH3 | – | – | –OH | – | – | |
Tangeretin | – | –OCH3 | –OCH3 | –OCH3 | –OCH3 | – | – | –OCH3 | – | – | |
| Serpyllin | – | –OH | – | –OCH3 | –OCH3 | –OCH3 | –OCH3 | –OCH3 | – | – | |
| Sudachitin | – | –OH | –OCH3 | –OH | –OCH3 | – | –OCH3 | –OH | – | – | |
| Acerosin | – | –OH | –OCH3 | –OH | –OCH3 | – | –OH | –OCH3 | – | – | |
| Hymenoxin | – | –OH | –OCH3 | –OH | –OCH3 | – | –OCH3 | –OCH3 | – | – | |
| Gardenin D | – | –OH | –OCH3 | –OCH3 | –OCH3 | – | –OH | –OCH3 | – | – | |
Nobiletin | – | –OCH3 | –OCH3 | –OCH3 | –OCH3 | – | –OCH3 | –OCH3 | – | – | |
| Scaposin | – | –OH | –OCH3 | –OH | –OCH3 | – | –OCH3 | –OCH3 | –OH | ||
| Name | Structure | R3 | R5 | R6 | R7 | R8 | R2' | R3' | R4' | R5' | R6' |
References
^ abcdefg "Flavonoids". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. November 2015. Retrieved 30 March 2018..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "Flavone". ChemSpider, Royal Society of Chemistry. 2015. Retrieved 30 March 2018.
^ Lotito, S; Frei, B (2006). "Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon?". Free Radical Biology and Medicine. 41 (12): 1727–46. doi:10.1016/j.freeradbiomed.2006.04.033. PMID 17157175.
^ David Stauth (5 March 2007). "Studies force new view on biology of flavonoids". EurekAlert!; Adapted from a news release issued by Oregon State University.
^ Cermak R, Wolffram S., The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms,Curr Drug Metab. 2006 Oct;7(7):729-44.
^ Si D, Wang Y, Zhou YH, et al. (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols". Drug Metab. Dispos. 37 (3): 629–34. doi:10.1124/dmd.108.023416. PMID 19074529.
[1]
^ Sarda SR, Pathan MY, Paike VV, Pachmase PR, Jadhav WN, Pawar RP (2006). "A facile synthesis of flavones using recyclable ionic liquid under microwave irradiation" (PDF). Arkivoc. xvi (16): 43–8. doi:10.3998/ark.5550190.0007.g05.
^ Wessely F, Moser GH (December 1930). "Synthese und Konstitution des Skutellareins". Monatsh. Chem. 56 (1): 97–105. doi:10.1007/BF02716040.
^ Larget R, Lockhart B, Renard P, Largeron M (April 2000). "A convenient extension of the Wessely-Moser rearrangement for the synthesis of substituted alkylaminoflavones as neuroprotective agents in vitro". Bioorg. Med. Chem. Lett. 10 (8): 835–8. doi:10.1016/S0960-894X(00)00110-4. PMID 10782697.
^ Harborne, Jeffrey B.; Marby, Helga; Marby, T. J. (1975). The Flavonoids - Springer. doi:10.1007/978-1-4899-2909-9. ISBN 978-0-12-324602-8.
External links
Flavones at the US National Library of Medicine Medical Subject Headings (MeSH)
