Phenolic acid
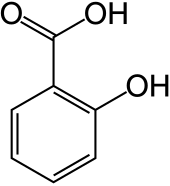
Chemical structure of salicylic acid, a common phenolic acid
Phenolic acids or phenolcarboxylic acids are types of aromatic acid compound. Included in that class are substances containing a phenolic ring and an organic carboxylic acid function (C6-C1 skeleton). Two important naturally occurring types of phenolic acids are hydroxybenzoic acids and hydroxycinnamic acids, which are derived from non-phenolic molecules of benzoic and cinnamic acid, respectively.[1]
Contents
1 Occurrences
2 Chemistry
3 See also
4 References
Occurrences
Phenolic acids can be found in many plant species. Their content in dried fruits can be high.
Natural phenols in horse grams (Macrotyloma uniflorum) are mostly phenolic acids, namely 3,4-dihydroxy benzoic, p-hydroxy benzoic, vanillic, caffeic, p-coumaric, ferulic, syringic, and sinapinic acids.[2]
Phenolic acids can be found in mushroom Basidiomycetes species.[3] It is also a part of the humic substances, which are the major organic constituents of soil humus.
Many phenolic acids can be found in human urine.[4]
Chemistry
Immobilized Candida antarctica lipase can be used to catalyze the direct acetylation of flavonoids with phenolic acids.[5]
See also
- Benzoic acid
- Aromatic alcohol
- List of phytochemicals in food
References
^ Heleno, Sandrina A.; Martins, Anabela; Queiroz, Maria João R. P.; Ferreira, Isabel C. F. R. (2015-04-15). "Bioactivity of phenolic acids: metabolites versus parent compounds: a review". Food Chemistry. 173: 501–513. doi:10.1016/j.foodchem.2014.10.057. ISSN 0308-8146. PMID 25466052..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Kawsar, S.M.A.; Huq, E.; Nahar, N.; Ozeki, Y. (2008). "Identification and Quantification of Phenolic Acids in Macrotyloma uniflorum by Reversed Phase-HPLC". American Journal of Plant Physiology. 3 (4): 165. doi:10.3923/ajpp.2008.165.172.
^ Barros, L.; Dueñas, M.; Ferreira, I. C.; Baptista, P.; Santos-Buelga, C. (June 2009). "Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species". Food and Chemical Toxicology. 47 (6): 1076–1079. doi:10.1016/j.fct.2009.01.039. PMID 19425182.
^ Armstrong, M. D.; Shaw, K. N.; Wall, P. E. (January 1, 1956). "The phenolic acids of human urine. Paper chromatography of phenolic acids" (pdf). The Journal of Biological Chemistry. 218 (1): 293–303. PMID 13278337.
^ Stevenson, David E.; Wibisono, Reginald; Jensen, Dwayne J.; Stanley, Roger A.; Cooney, Janine M. (3 October 2006). "Direct acylation of flavonoid glycosides with phenolic acids catalysed by Candida antarctica lipase B (Novozym 435®)". Enzyme and Microbial Technology. 39 (6): 1236–41. doi:10.1016/j.enzmictec.2006.03.006.