Chloride
| |||
| Names | |||
|---|---|---|---|
Systematic IUPAC name Chloride[1] | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
Beilstein Reference | 3587171 | ||
ChEBI |
| ||
ChEMBL |
| ||
ChemSpider |
| ||
Gmelin Reference | 14910 | ||
IUPHAR/BPS |
| ||
KEGG |
| ||
PubChem CID |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | Cl− | ||
Molar mass | 35.45 g·mol−1 | ||
Conjugate acid | Hydrogen chloride | ||
| Thermochemistry | |||
Std molar entropy (S | 153.36 J K−1 mol−1[2] | ||
Std enthalpy of formation (ΔfH | −167 kJ·mol−1[2] | ||
| Related compounds | |||
Other anions | Fluoride Bromide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
The chloride ion /ˈklɔːraɪd/[3] is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.[4] It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating fluid in and out of cells. Less frequently, the word chloride may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion.
Contents
1 Electronic properties
2 Occurrence in nature
3 Role in commerce
3.1 Water quality and processing
3.2 Domestic uses
3.3 Corrosion
4 Role in biology
5 Reactions of chloride
6 Examples
7 Other oxyanions
8 See also
9 References
Electronic properties
A chloride ion is much larger than a chlorine atom, 167 and 99 pm, respectively. The ion is colorless and diamagnetic. In aqueous solution, it is highly soluble in most cases; however, some chloride salts, such as silver chloride, lead(II) chloride, and mercury(I) chloride are slightly soluble in water.[5] In aqueous solution, chloride is bound by the protic end of the water molecules.
Occurrence in nature
Sea water contains 1.94% chloride. Some chloride-containing minerals include the chlorides of sodium (halite or NaCl), potassium (sylvite or KCl), and magnesium (bischofite), hydrated MgCl2. The concentration of chloride in the blood is called serum chloride, and this concentration is regulated by the kidneys. A chloride ion is a structural component of some proteins, e.g., it is present in the amylase enzyme.
Role in commerce
The chlor-alkali industry is a major consumer of the world's energy budget. This process converts sodium chloride into chlorine and sodium hydroxide, which are used to make many other materials and chemicals. The process involves two parallel reactions:
- 2 Cl− → Cl
2 + 2 e−
- 2 H
2O + 2 e− → H2 + 2 OH−
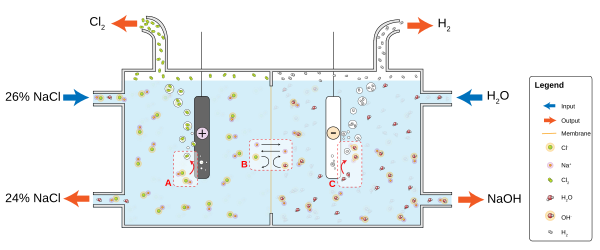
Basic membrane cell used in the electrolysis of brine. At the anode (A), chloride (Cl−) is oxidized to chlorine. The ion-selective membrane (B) allows the counterion Na+ to freely flow across, but prevents anions such as hydroxide (OH−) and chloride from diffusing across. At the cathode (C), water is reduced to hydroxide and hydrogen gas.
Water quality and processing
Another major application involving chloride is desalination, which involves the energy intensive removal of chloride salts to give potable water. In the petroleum industry, the chlorides are a closely monitored constituent of the mud system. An increase of the chlorides in the mud system may be an indication of drilling into a high-pressure saltwater formation. Its increase can also indicate the poor quality of a target sand.[citation needed]
Chloride is also a useful and reliable chemical indicator of river / groundwater fecal contamination, as chloride is a non-reactive solute and ubiquitous to sewage & potable water. Many water regulating companies around the world utilize chloride to check the contamination levels of the rivers and potable water sources.[6]
Domestic uses
Chloride salts such as sodium chloride are used to preserve food.
Corrosion
The presence of chlorides, e.g. in seawater, significantly aggravates the conditions for pitting corrosion of most metals (including stainless steels, aluminum, aluminum alloys, and high-alloyed materials) by enhancing the formation and growth of the pits through an autocatalytic process.

Crystals of sodium chloride, which, like most chloride salts is colorless and water-soluble.

The structure of sodium chloride, revealing the tendency of chloride ions (green spheres) to link to several cations.
Role in biology
Chloride is an essential electrolyte, trafficking in and out of cells through chloride channels and playing a key role in maintaining cell homeostasis and transmitting action potentials in neurons.[7] Characteristic concentrations of chloride in model organisms are: in both E. coli and budding yeast are 10-200mM (media dependent), in mammalian cell 5-100mM and in blood plasma 100mM.[8]
Reactions of chloride
Chloride can be oxidized but not reduced. The first oxidation, as employed in the chlor-alkali process, is conversion to chlorine gas. Chlorine can be further oxidized to other oxides and oxyanions including hypochlorite (ClO−, the active ingredient in chlorine bleach), chlorine dioxide (ClO2), chlorate (ClO−
3), and perchlorate (ClO−
4).
In terms of its acid–base properties, chloride is a very weak base as indicated by the negative value of the pKa of hydrochloric acid. Chloride can be protonated by strong acids, such as sulfuric acid:
- NaCl + H2SO4 → NaHSO4 + HCl
Ionic chloride salts reaction with other salts to exchange anions. The presence of chloride is often detected by its formation of an insoluble silver chloride upon treatment with silver ion:
- Cl− + Ag+ → AgCl
The concentration of chloride in an assay can be determined using a chloridometer, which detects silver ions once all chloride in the assay has precipitated via this reaction.
Chlorided silver electrodes are commonly used in ex vivo electrophysiology. [9]
Examples
An example is table salt, which is sodium chloride with the chemical formula NaCl. In water, it dissociates into Na+ and Cl− ions. Salts such as calcium chloride, magnesium chloride, potassium chloride have varied uses ranging from medical treatments to cement formation.[4]
Calcium chloride (CaCl2) is a salt that is marketed in pellet form for removing dampness from rooms. Calcium chloride is also used for maintaining unpaved roads and for fortifying roadbases for new construction. In addition, calcium chloride is widely used as a de-icer, since it is effective in lowering the melting point when applied to ice.[10]
Examples of covalently bonded chlorides are phosphorus trichloride, phosphorus pentachloride, and thionyl chloride, all three of which are reactive chlorinating reagents that have been used in a laboratory.
Other oxyanions
Chlorine can assume oxidation states of −1, +1, +3, +5, or +7. Several neutral chlorine oxides are also known.
| Chlorine oxidation state | −1 | +1 | +3 | +5 | +7 |
|---|---|---|---|---|---|
| Name | chloride | hypochlorite | chlorite | chlorate | perchlorate |
| Formula | Cl− | ClO− | ClO− 2 | ClO− 3 | ClO− 4 |
| Structure |  |  |  |
See also
Halide (compounds of halogens)- Renal chloride reabsorption
References
^ "Chloride ion - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ ab Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A21. ISBN 0-618-94690-X.
^ Wells, John C. (2008), Longman Pronunciation Dictionary (3rd ed.), Longman, p. 143, ISBN 9781405881180.
^ ab Green, John, and Sadru Damji. "Chapter 3." Chemistry. Camberwell, Vic.: IBID, 2001. Print.
^ Zumdahl, Steven (2013). Chemical Principles (7th ed.). Cengage Learning. p. 109. ISBN 978-1-285-13370-6.
^ "Chlorides". www.gopetsamerica.com. Retrieved 14 April 2018.
^ Jentsch, Thomas J.; Stein, Valentin; Weinreich, Frank; Zdebik, Anselm A. (2002-04-01). "Molecular Structure and Physiological Function of Chloride Channels". Physiological Reviews. 82 (2): 503–568. doi:10.1152/physrev.00029.2001. ISSN 0031-9333. PMID 11917096.
^ Milo, Ron; Philips, Rob. "Cell Biology by the Numbers: What are the concentrations of different ions in cells?". book.bionumbers.org. Retrieved 24 March 2017.
^ Molleman, Areles (2003). "Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology". Wiley & Sons.
ISBN 978-0-471-48685-5.
^ "Common Salts". hyperphysics.phy-astr.gsu.edu. Georgia State University.



