Potassium chloride
 | |
 | |
| Names | |
|---|---|
| Other names Sylvite Muriate of potash | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChEMBL |
|
ChemSpider |
|
DrugBank |
|
ECHA InfoCard | 100.028.374 |
E number | E508 (acidity regulators, ...) |
KEGG |
|
PubChem CID |
|
RTECS number | TS8050000 |
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | KCl |
Molar mass | 74.5513 g·mol−1 |
| Appearance | white crystalline solid |
Odor | odorless |
Density | 1.984 g/cm3 |
Melting point | 770 °C (1,420 °F; 1,040 K) |
Boiling point | 1,420 °C (2,590 °F; 1,690 K) |
Solubility in water | 217.1 g/L (0 °C) 253.9 g/L (20 °C) 360.5 g/L (100 °C) |
Solubility | Soluble in glycerol, alkalies Slightly soluble in alcohol Insoluble in ether[1] |
Acidity (pKa) | ~7 |
Magnetic susceptibility (χ) | −39.0·10−6 cm3/mol |
Refractive index (nD) | 1.4902 (589 nm) |
| Structure | |
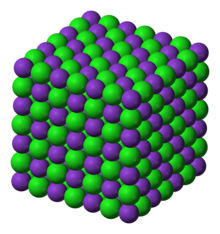
Crystal structure | face centered cubic |
Space group | Fm3m, No. 225 |
Lattice constant | a = 629.2 pm[2] |
Coordination geometry | Octahedral (K+) Octahedral (Cl−) |
| Thermochemistry | |
Std molar entropy (S | 83 J·mol−1·K−1[3] |
Std enthalpy of formation (ΔfH | −436 kJ·mol−1[3] |
| Pharmacology | |
ATC code | A12BA01 (WHO) B05XA01 (WHO) |
Pregnancy category |
|
Routes of administration | Oral, IV, IM |
Pharmacokinetics: | |
Excretion | Renal: 90%; Fecal: 10%[4] |
| Hazards | |
Safety data sheet | ICSC 1450 |
NFPA 704 |  0 1 0 |
Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 2600 mg/kg (oral, rat)[5] |
| Related compounds | |
Other anions | Potassium fluoride Potassium bromide Potassium iodide Potassium astatide |
Other cations | Lithium chloride Sodium chloride Rubidium chloride Caesium chloride Francium chloride |
Related compounds | Potassium chlorate Potassium perchlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Potassium chloride (KCl) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water and its solutions have a salt-like taste. KCl is used as a fertilizer,[6] in medicine, in scientific applications, and in food processing, where it may be known as E number additive E508.
In a few states of the United States it is used to cause cardiac arrest as the third drug in the "three drug cocktail" for executions by lethal injection. It occurs naturally as the mineral sylvite, and in combination with sodium chloride as sylvinite.[7]
The version for injection is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[8]
Contents
1 Uses
1.1 Fertilizer
1.2 Medical use
1.3 Industrial
2 Side effects
3 Chemical properties
3.1 Solubility
3.2 Redox and the conversion to potassium metal
4 Physical properties
5 Production
5.1 Laboratory methods
6 References
7 Further reading
Uses
Fertilizer
The majority of the potassium chloride produced is used for making fertilizer, called potash, since the growth of many plants is limited by potassium availability. The two main types of potash are: Muriate of Potash (MOP, Potassium Chloride) and Sulphate of Potash (SOP, Potassium Sulphate). While SOP typically sells at a premium to MOP, the vast majority of potash fertilizer worldwide is sold as MOP.
Medical use
Potassium is vital in the human body, and potassium chloride by mouth is the common means to treat low blood potassium, although it can also be given intravenously. It can be used as a salt substitute for food, but due to its weak, bitter, unsalty flavor, it is usually mixed with ordinary table salt (sodium chloride) to improve the taste. The addition of 1 ppm of thaumatin considerably reduces this bitterness.[9] Complaints of bitterness or a chemical or metallic taste are also reported with potassium chloride used in food.[10]
Industrial
As a chemical feedstock, it is used for the manufacture of potassium hydroxide and potassium metal. It is also used in medicine, lethal injections, scientific applications, food processing, soaps, and as a sodium-free substitute for table salt for people concerned about the health effects of sodium.
It is used as a supplement in animal feed to boost the amount of nutrients in the feed, which in turn promotes healthy growth in animals. As an added benefit, it is known to increase milk production.
It is sometimes used in water as a completion fluid in petroleum and natural gas operations, as well as being an alternative to sodium chloride in household water softener units.
Glass manufactures use granular potash as a flux, lowering the temperature at which a mixture melts. Because potash confers excellent clarity to glass, it is commonly used in eyeglasses, glassware, televisions and computer monitors.
KCl is useful as a beta radiation source for calibration of radiation monitoring equipment, because natural potassium contains 0.0118% of the isotope 40K. One kilogram of KCl yields 16350 becquerels of radiation consisting of 89.28% beta and 10.72% gamma with 1.46083 MeV.
Potassium chloride is used in some de-icing products that are designed to be safer for pets and plants, though these are inferior in melting quality to calcium chloride [lowest usable temperature 12 °F (−11 °C) v. −25 °F (−32 °C)]. It is also used in various brands of bottled water, as well as in bulk quantities for fossil fuel drilling purposes.
Potassium chloride was once used as a fire extinguishing agent, used in portable and wheeled fire extinguishers. Known as Super-K dry chemical, it was more effective than sodium bicarbonate-based dry chemicals and was compatible with protein foam. This agent fell out of favor with the introduction of potassium bicarbonate (Purple-K) dry chemical in the late 1960s, which was much less corrosive and more effective. It is rated for B and C fires.
Along with sodium chloride and lithium chloride, potassium chloride is used as a flux for the gas welding of aluminium.
Potassium chloride is also an optical crystal with a wide transmission range from 210 nm to 20 µm. While cheap, KCl crystal is hygroscopic. This limits its application to protected environments or short-term uses such as prototyping. Exposed to free air, KCl optics will "rot". Whereas KCl components were formerly used for infrared optics, it has been entirely replaced by much tougher crystals such as zinc selenide.
Potassium chloride has also been used to produce heat packs which employ exothermic chemical reactions,[11] but these have mostly been discontinued with the advent of cheaper and more efficient methods, such as the oxidation of metals ('Hot Hands' one-time-use products) or the crystallization of sodium acetate (multiple-use products).[citation needed]
Potassium chloride is used as a scotophor with designation P10 in dark-trace CRTs, e.g. in the Skiatron.
Side effects
The typical amounts of potassium chloride found in the diet appear to be generally safe.[12] In larger quantities, however, potassium chloride is toxic. The LD50 of orally ingested potassium chloride is approximately 2.5 g/kg, or 190 grams (6.7 oz) for a body mass of 75 kilograms (165 lb). In comparison, the LD50 of sodium chloride (table salt) is 3.75 g/kg.
Intravenously, the LD50 of potassium chloride is far smaller, at about 57.2 mg/kg to 66.7 mg/kg; this is found by dividing the lethal concentration of positive potassium ions (about 30 to 35 mg/kg)[13] by the proportion by mass of potassium ions in potassium chloride (about .52445 mg K+/mg KCl)[14]. In such quantities, it has severe consequences on the cardiac muscles, potentially causing cardiac arrest and rapid death. For this reason, it is used as the third and final drug delivered in the lethal injection process.
Chemical properties
Solubility
KCl is soluble in a variety of polar solvents.
| Solvent | Solubility (g/kg of solvent at 25 °C) |
|---|---|
| H2O | 360 |
| Liquid ammonia | 0.4 |
| Liquid sulfur dioxide | 0.41 |
| Methanol | 5.3 |
| Formic acid | 192 |
| Sulfolane | 0.04 |
| Acetonitrile | 0.024 |
| Acetone | 0.00091 |
| Formamide | 62 |
| Acetamide | 24.5 |
| Dimethylformamide | 0.17–0.5 |
Solutions of KCl are common standards, for example for calibration of the electrical conductivity of (ionic) solutions, since KCl solutions are stable, allowing for reproducible measurements. In aqueous solution, it is essentially fully ionized into solvated K+ and Cl– ions.
Redox and the conversion to potassium metal
Although potassium is more electropositive than sodium, KCl can be reduced to the metal by reaction with metallic sodium at 850 °C because the more volatile potassium can be removed by distillation (see Le Chatelier's principle):
- KCl(l) + Na(l) ⇌ NaCl(l) + K(g)
This method is the main method for producing metallic potassium. Electrolysis (used for sodium) fails because of the high solubility of potassium in molten KCl.[7]
Physical properties

"Raise banana yields using Israeli potassium chloride!", an ad above a highway in a banana-growing district of Hekou County, Yunnan, China
The crystal structure of potassium chloride is like that of NaCl. It adopts a face-centered cubic structure. Its lattice constant is roughly 6.3 Å. Crystals cleave easily in three directions.
Some other properties are
- Transmission range: 210 nm to 20 µm
Transmittivity = 92% at 450 nm and rises linearly to 94% at 16 µm- Refractive index = 1.456 at 10 µm
- Reflection loss = 6.8% at 10 µm (two surfaces)
- dN/dT (expansion coefficient)= −33.2×10−6/°C
- dL/dT (refractive index gradient)= 40×10−6/°C
- Thermal conductivity = 0.036 W/(cm·K)
- Damage threshold (Newman and Novak): 4 GW/cm2 or 2 J/cm2 (0.5 or 1 ns pulse rate); 4.2 J/cm2 (1.7 ns pulse rate Kovalev and Faizullov)
As with other compounds containing potassium, KCl in powdered form gives a lilac flame.
Production

Sylvite

Sylvinite
Potassium chloride is extracted from minerals sylvite, carnallite, and potash. It is also extracted from salt water and can be manufactured by crystallization from solution, flotation or electrostatic separation from suitable minerals. It is a by-product of the production of nitric acid from potassium nitrate and hydrochloric acid.
The vast majority of potassium chloride is produced as agricultural and industrial grade potash in Saskatchewan, Canada, as well as Russia and Belarus. Saskatchewan alone accounts for over 25% of the world's potash production in 2017.[16]
Laboratory methods
Potassium chloride is inexpensively available and is rarely prepared intentionally in the laboratory. It can be generated by treating potassium hydroxide (or other potassium bases) with hydrochloric acid:
- KOH + HCl → KCl + H2O
This conversion is an acid-base neutralization reaction. The resulting salt can then be purified by recrystallization. Another method would be to allow potassium to burn in the presence of chlorine gas, also a very exothermic reaction:
- 2 K + Cl2 → 2 KCl
References
^ "Potassium chloride (PIM 430)". International Programme on Chemical Safety. 3.3.1 Properties of the substance. Retrieved 2011-01-17..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ D.B. Sirdeshmukh; L. Sirdeshmukh; K.G. Subhadra. Alkali Halides: A Handbook of Physical Properties.
^ ab Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 0-618-94690-X.
^ "Compound Summary for CID 4873". Pubchem.ncbi.nlm.nih.gov. Retrieved 17 October 2015.
^ Chambers, Michael. "ChemIDplus - 7447-40-7 - WCUXLLCKKVVCTQ-UHFFFAOYSA-M - Potassium chloride [USP:JAN] - Similar structures search, synonyms, formulas, resource links, and other chemical information". Chem.sis.nlm.nih.gov. Retrieved 22 December 2017.
^ "Potassium Fertilizers (Penn State Agronomy Guide)". Penn State Agronomy Guide (Penn State Extension). Retrieved 2016-12-10.
^ ab Burkhardt, Elizabeth R. (2006). "Potassium and Potassium Alloys". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a22_031.pub2.
^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Retrieved 8 December 2016.
^ Lorient, Denis; Linden, G. (1999). New ingredients in food processing: biochemistry and agriculture. Boca Raton: CRC Press. p. 357. ISBN 1-85573-443-5.... in dietary food containing potassium chloride, thaumatin added in the ratio of 1 ppm considerably reduces the sensation of bitterness. ...
^ Sinopoli, Dominique A.; Lawless, Harry T. (2012). "Taste Properties of Potassium Chloride Alone and in Mixtures with Sodium Chloride Using a Check-All-That-Apply Method". Journal of Food Science. 77 (9): S319–22. doi:10.1111/j.1750-3841.2012.02862.x. PMID 22901084.
^ U.S. Patent 3,874,504
^ Nutrition, Center for Food Safety and Applied. "GRAS Substances (SCOGS) Database - Select Committee on GRAS Substances (SCOGS) Opinion: Potassium chloride". www.fda.gov. Retrieved 21 June 2017.
^ "Fatal poisoning by potassium in human and rabbit". Forensic Science. 9: 33–36. 1977-01-01. doi:10.1016/0300-9432(77)90062-0. ISSN 0300-9432.
^ "Molecular weight of KCl". www.convertunits.com. Retrieved 2018-11-04.
^ Burgess, J. (1978). Metal Ions in Solution. New York: Ellis Horwood. ISBN 0-85312-027-7.
[page needed]
^ "Potash Mineral Commodity Summaries 2018" (PDF).
Further reading
Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 0-08-022057-6.
