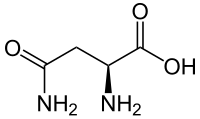
Asparagine
 | |
 | |
| Names | |
|---|---|
IUPAC name Asparagine | |
| Other names 2-Amino-3-carbamoylpropanoic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChEMBL |
|
ChemSpider |
|
DrugBank |
|
ECHA InfoCard | 100.000.669 |
EC Number | 200-735-9 |
IUPHAR/BPS |
|
KEGG |
|
PubChem CID |
|
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C4H8N2O3 |
Molar mass | 132.12 g·mol−1 |
| Appearance | white crystals |
Density | 1.543 g/cm3 |
Melting point | 234 °C (453 °F; 507 K) |
Boiling point | 438 °C (820 °F; 711 K) |
Solubility in water | 2.94 g/100 mL |
Solubility | soluble in acids, bases, negligible in methanol, ethanol, ether, benzene |
log P | −3.82 |
Acidity (pKa) | 2.02 (carboxyl), 8.80 (amino)[1] |
Magnetic susceptibility (χ) | -69.5·10−6 cm3/mol |
| Structure | |
Crystal structure | orthorhombic |
| Thermochemistry | |
Std enthalpy of formation (ΔfH | −789.4 kJ/mol |
| Hazards | |
Safety data sheet | See: data page Sigma-Alrich |
NFPA 704 |  0 1 0 |
Flash point | 219 °C (426 °F; 492 K) |
Supplementary data page | |
Structure and properties | Refractive index (n), Dielectric constant (εr), etc. |
Thermodynamic data | Phase behaviour solid–liquid–gas |
Spectral data | UV, IR, NMR, MS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Asparagine (symbol Asn or N[2]), is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC.
A reaction between asparagine and reducing sugars or other source of carbonyls produces acrylamide in food when heated to sufficient temperature. These products occur in baked goods such as French fries, potato chips, and toasted bread.
Contents
1 History
2 Structural function in proteins
3 Sources
3.1 Dietary sources
3.2 Biosynthesis
4 Degradation
5 Function
6 Zwitterion structure
7 Alleged cancer link in laboratory mice
8 References
9 External links
History
Asparagine was first isolated in 1806 in a crystalline form by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet (then a young assistant) from asparagus juice,[3][4] in which it is abundant, hence the chosen name. It was the first amino acid to be isolated.
Three years later, in 1809, Pierre Jean Robiquet identified a substance from liquorice root with properties which he qualified as very similar to those of asparagine,[5] and which Plisson identified in 1828 as asparagine itself.[6][7]
The determination of asparagine's structure required decades of research. The empirical formula for asparagine was first determined in 1833 by the French chemists Antoine François Boutron Charlard and Théophile-Jules Pelouze; in the same year, the German chemist Justus Liebig provided a more accurate formula.[8][9] In 1846 the Italian chemist Raffaele Piria treated asparagine with nitrous acid, which removed the molecule's amine (–NH2) groups and transformed asparagine into malic acid.[10] This revealed the molecule's fundamental structure: a chain of four carbon atoms. Piria thought that asparagine was a diamide of malic acid;[11] however, in 1862 the German chemist Hermann Kolbe showed that this surmise was wrong; instead, Kolbe concluded that asparagine was an amide of an amine of succinic acid.[12] In 1886, the Italian chemist Arnaldo Piutti (1857–1928) discovered a mirror image or "enantiomer" of the natural form of asparagine, which shared many of asparagine's properties, but which also differed from it.[13] Since the structure of asparagine was still not fully known – the location of the amine group within the molecule was still not settled[14] – Piutti synthesized asparagine and thus determined its true structure.[15]
Structural function in proteins
Since the asparagine side-chain can form hydrogen bond interactions with the peptide backbone, asparagine residues are often found near the beginning of alpha-helices as asx turns and asx motifs, and in similar turn motifs, or as amide rings, in beta sheets. Its role can be thought as "capping" the hydrogen bond interactions that would otherwise be satisfied by the polypeptide backbone.
Asparagine also provides key sites for N-linked glycosylation, modification of the protein chain with the addition of carbohydrate chains. Typically, a carbohydrate tree can solely be added to an asparagine residue if the latter is flanked on the C side by X-serine or X-threonine, where X is any amino acid with the exception of proline.[16]
Sources
Dietary sources
Asparagine is not essential for humans, which means that it can be synthesized from central metabolic pathway intermediates and is not required in the diet.
Asparagine is found in:
Animal sources: dairy, whey, beef, poultry, eggs, fish, lactalbumin, seafood
Plant sources: asparagus, potatoes, legumes, nuts, seeds, soy, whole grains
Biosynthesis
The precursor to asparagine is oxaloacetate. Oxaloacetate is converted to aspartate using a transaminase enzyme. The enzyme transfers the amino group from glutamate to oxaloacetate producing α-ketoglutarate and aspartate. The enzyme asparagine synthetase produces asparagine, AMP, glutamate, and pyrophosphate from aspartate, glutamine, and ATP. In the asparagine synthetase reaction, ATP is used to activate aspartate, forming β-aspartyl-AMP. Glutamine donates an ammonium group, which reacts with β-aspartyl-AMP to form asparagine and free AMP.

Degradation
Asparagine usually enters the citric acid cycle in humans as oxaloacetate.[citation needed] In bacteria, the degradation of asparagine leads to the production of oxaloacetate which is the molecule which combines with citrate in the citric acid cycle (Krebs cycle). Asparagine is hydrolyzed to aspartate by asparaginase. Aspartate then undergoes transamination to form glutamate and oxaloacetate from alpha-ketoglutarate.
Function
Asparagine is required for development and function of the brain.[17] It also plays an important role in the synthesis of ammonia.[citation needed]
The addition of N-acetylglucosamine to asparagine is performed by oligosaccharyltransferase enzymes in the endoplasmic reticulum.[18] This glycosylation is important both for protein structure[19] and protein function.[20]
Zwitterion structure

(S)-Asparagine (left) and (R)-asparagine (right) in zwitterionic form at neutral pH.
Alleged cancer link in laboratory mice
According to a 2018 article in The Guardian, a study found that decreasing levels of asparagine "dramatically" reduced the spread of breast cancer in laboratory mice.[21][22] The article noted that similar studies had not been conducted in humans.
References
^ R. M. C. Dawson; Daphne Elliott; W. H. Elliott; K. M. Jones. Clarendon, eds. (1959). Data for Biochemical Research. Oxford: Clarendon Press. OCLC 644267041..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
^ Vauquelin LN, Robiquet PJ (1806). "La découverte d'un nouveau principe végétal dans le suc des asperges". Annales de Chimie (in French). 57: 88–93. hdl:2027/nyp.33433062722578.
^ R.H.A. Plimmer (1912) [1908]. R.H.A. Plimmer; F.G. Hopkins, eds. The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co. p. 112. Retrieved January 18, 2010.
^ Robiquet, P.J. (1809). "Analyse de la racine de réglisse" [Analysis of licorice root]. Annales de Chimie et de Physique (in French). 72 (1): 143–159.
^ Plisson, A. (1828). "De l'indentité de l'asparagine avec l'agédoïte" [On the identity of asparagine with agédoïte]. Journal de Pharmacie et des Sciences Accessoires (in French). 14 (4): 177–182.
^ Harvey Wickes Felter, M.D. & John Uri Lloyd (1898). "Glycyrrhiza (U. S. P.)—Glycyrrhiza". King's American Dispensatory. Henriette's Herbal Homepage.
^ Boutron-Charlard; Pelouze (1833). "Ueber das Asparamid (Asparagin des Herrn Robiquet) und die Asparamidsäure" [On asparamide (the asparagine of Mr. Robiquet) and aspartic acid]. Annalen der Chemie (in German). 6: 75–88. The empirical formula of asparagine appears on p. 80.
^ Liebig, Justus (1833). "Ueber die Zusammensetzung des Asparamids und der Asparaginsäure" [On the composition of asparamide [asparagine] and aspartic acid]. Annalen der Chemie (in German). 7: 146–150. The empirical formula appears on p. 149 ; the formula is correct if the subscripts are divided by 2.
^ See:
Piria, Raffaele (January 1846). "Studi sulla costituzione chimica dell' asparagina e dell' acido aspartico" [Studies of the chemical constitution of asparagine and aspartic acid]. Il Cimento (in Italian). 4: 55–73.
- French translation: Piria, Raffaele (1848). "Recherches sur la constitution chimique de l'asparagine et de l'acide aspartique" [Investigations into the chemical constitution of asparagine and of aspartic acid]. Annales de Chimie et de Physique. 3rd series (in French). 22: 160–179. From p. 175: " … on voit, en outre, que l'asparagine et l'acide aspartique lui-même se décomposent avec une facilité remarquable, sous l'influence de l'acide hyponitrique, en fournissant du gaz azote et de l'acide malique." ( … one sees, in addition, that asparagine and aspartic acid itself are decomposed with a remarkable ease under the influence of nitrous acid, rendering nitrogen gas and malic acid.)
^ Plimmer, Robert Henry Aders (1912). The Chemical Constitution of the Proteins. Part I: Analysis (2nd ed.). London, England: Longmans, Green and Co. p. 112.
^ Kolbe, Hermann (1862). "Ueber die chemische Constitution des Asparagins und der Asparaginsäure" [On the chemical constitution of asparagine and aspartic acid]. Annalen der Chemie (in German). 121: 232–236.
^ Piutti, A. (1886). "Ein neues Asparagin" [A new asparagine]. Berichte der Deutschen Chemischen Gesellschaft (in German). 19: 1691–1695.
^ The French chemist Edouard Grimaux thought that the amine group (–NH2) was located next to the amide group (–C(O)NH2), whereas the Italian chemist Icilio Guareschi thought that the amine group was located next to the carboxyl group (–COOH).
Grimaux, Edouard (1875). "Recherches synthétiques sur le groupe urique" [Synthetic investigations of the uric group]. Bulletin de la Société Chimique de Paris. 2nd series (in French). 24: 337–355. On p. 352, Grimaux presented two putative structures for asparagine, and on p. 353, he favored structure (I.), which is incorrect. From p. 353: " … ce sont les formules marquées du chiffre I qui me semblent devoir être adoptées pour l'asparagine, … " ( … it is the formulas marked by the figure I which, it seems to me, should be adopted for asparagine, … )
Guareschi, Icilio (1876). "Studi sull' asparagine e sull' acido aspartico" [Studies of asparagine and of aspartic acid]. Atti della Reale Academia del Lincei. 2nd series (in Italian). 3 (pt. 2): 378–393. On p. 388, Guareschi proposed two structures (α and β) for asparagine; he favored α, the correct one. From p. 388: "La formola α mi sembra preferibile per seguente ragione: … " (The formula α seems preferable to me for the following reason: … )- English abstract in: Guareschi, J. (1877). "Asparagine and aspartic acid". Journal of the Chemical Society. 31: 457–459. See especially p. 458.
^ Piutti, Arnaldo (1888). "Sintesi e costituzione delle asparagine" [Synthesis and constitution of asparagine]. Gazzetta Chimica Italiana (in Italian). 18: 457–472.
^ Brooker, Robert; Widmaier, Eric; Graham, Linda; Stiling, Peter; Hasenkampf, Clare; Hunter, Fiona; Bidochka, Michael; Riggs, Daniel (2010). "Chapter 5: Systems Biology of Cell Organization". Biology (Canadian ed.). United States of America: McGraw-Hill Ryerson. pp. 105–106. ISBN 978-0-07-074175-1.
^ Ruzzo, EK; et, al (2013). "Deficiency of asparagine synthetase causes congenital microcephaly and a progressive form of encephalopathy". Neuron. 80 (2): 429–41. doi:10.1016/j.neuron.2013.08.013. PMC 3820368. PMID 24139043.
^ Burda, Patricie; Aebi, Markus (1999). "The dolichol pathway of N-linked glycosylation". Biochimica et Biophysica Acta (BBA) - General Subjects. 1426 (2): 239–257. doi:10.1016/S0304-4165(98)00127-5.
^ Imperiali, Barbara; o’Connor, Sarah E (1999). "Effect of N-linked glycosylation on glycopeptide and glycoprotein structure". Current Opinion in Chemical Biology. 3 (6): 643–9. doi:10.1016/S1367-5931(99)00021-6. PMID 10600722.
^ Patterson, Marc C. (2005). "Metabolic Mimics: The Disorders of N-Linked Glycosylation". Seminars in Pediatric Neurology. 12 (3): 144–51. doi:10.1016/j.spen.2005.10.002. PMID 16584073.
^ Sample, Ian (2018-02-07). "Spread of breast cancer linked to compound in asparagus and other foods". The Guardian. Retrieved 2018-05-30.
^ Hannon, Gregory J.; et al. (2018-02-07). "Asparagine bioavailability governs metastasis in a model of breast cancer". Nature. 554 (7692): 378–381. doi:10.1038/nature25465. PMC 5898613. PMID 29414946. Retrieved 2018-05-30.CS1 maint: Explicit use of et al. (link)
External links
- GMD MS Spectrum
- Why Asparagus Makes Your Pee Stink
