Neutrophil
| Neutrophil | |
|---|---|
 3D rendering of a neutrophil. | |
 Neutrophils with segmented nuclei surrounded by erythrocytes and platelets. Intra-cellular granules are visible in the cytoplasm (Giemsa stained). | |
| Details | |
| System | Immune system |
| Function | Granulocyte |
| Identifiers | |
| MeSH | D009504 |
| TH | H2.00.04.1.02012 |
Anatomical terms of microanatomy [edit on Wikidata] | |
Neutrophils (also known as neutrocytes) are the most abundant type of granulocytes and the most abundant (40% to 70%) type of white blood cells in most mammals. They form an essential part of the innate immune system. Their functions vary in different animals.[1]
They are formed from stem cells in the bone marrow and differentiated into subpopulations of neutrophil-killers and neutrophil-cagers. They are short-lived and highly motile, or mobile, as they can enter parts of tissue where other cells/molecules cannot. Neutrophils may be subdivided into segmented neutrophils and banded neutrophils (or bands). They form part of the polymorphonuclear cells family (PMNs) together with basophils and eosinophils.[2][3][4]
The name neutrophil derives from staining characteristics on hematoxylin and eosin (H&E) histological or cytological preparations. Whereas basophilic white blood cells stain dark blue and eosinophilic white blood cells stain bright red, neutrophils stain a neutral pink. Normally, neutrophils contain a nucleus divided into 2–5 lobes.
Neutrophils are a type of phagocyte and are normally found in the bloodstream. During the beginning (acute) phase of inflammation, particularly as a result of bacterial infection, environmental exposure,[5] and some cancers,[6][7] neutrophils are one of the first-responders of inflammatory cells to migrate towards the site of inflammation. They migrate through the blood vessels, then through interstitial tissue, following chemical signals such as Interleukin-8 (IL-8), C5a, fMLP, Leukotriene B4 and H2O2[8] in a process called chemotaxis. They are the predominant cells in pus, accounting for its whitish/yellowish appearance.[9]
Neutrophils are recruited to the site of injury within minutes following trauma and are the hallmark of acute inflammation;[10] however, due to some pathogens being indigestible, they can be unable to resolve certain infections without the assistance of other types of immune cells.
Contents
1 Structure
2 Development
2.1 Life span
3 Function
3.1 Chemotaxis
3.2 Anti-microbial function
3.3 Phagocytosis
3.4 Degranulation
3.5 Neutrophil extracellular traps
4 Clinical significance
5 Neutrophil antigens
6 Subpopulations
7 Video
8 Additional images
9 References
10 External links
Structure

Neutrophil granulocyte migrates from the blood vessel to the matrix, secreting proteolytic enzymes, in order to dissolve intercellular connections (to the improvement of its mobility) and envelop bacteria through phagocytosis.

Hypersegmented neutrophil.
When adhered to a surface, neutrophil granulocytes have an average diameter of 12–15 micrometers (µm) in peripheral blood smears. In suspension, human neutrophils have an average diameter of 8.85 µm.[11]
With the eosinophil and the basophil, they form the class of polymorphonuclear cells, named for the nucleus' multilobulated shape (as compared to lymphocytes and monocytes, the other types of white cells). The nucleus has a characteristic lobed appearance, the separate lobes connected by chromatin. The nucleolus disappears as the neutrophil matures, which is something that happens in only a few other types of nucleated cells.[12]:168 In the cytoplasm, the Golgi apparatus is small, mitochondria and ribosomes are sparse, and the rough endoplasmic reticulum is absent.[12]:170 The cytoplasm also contains about 200 granules, of which a third are azurophilic.[12]:170
Neutrophils are sexually dimorphic. Neutrophils from females exhibit a small additional X chromosome structure, known as a "neutrophil drumstick".[12]:174
Neutrophils will show increasing segmentation (many segments of the nucleus) as they mature. A normal neutrophil should have 3–5 segments. Hypersegmentation is not normal but occurs in some disorders, most notably vitamin B12 deficiency. This is noted in a manual review of the blood smear and is positive when most or all of the neutrophils have 5 or more segments.

Reference ranges for blood tests of white blood cells, comparing neutrophil amount (shown in pink) with that of other cells.
Neutrophils are the most abundant white blood cells in humans (approximately 1011 are produced daily); they account for approximately 50–70% of all white blood cells (leukocytes). The stated normal range for human blood counts varies between laboratories, but a neutrophil count of 2.5–7.5 x 109/L is a standard normal range. People of African and Middle Eastern descent may have lower counts, which are still normal.[13] A report may divide neutrophils into segmented neutrophils and bands.
When circulating in the bloodstream and inactivated, neutrophils are spherical. Once activated, they change shape and become more amorphous or amoeba-like and can extend pseudopods as they hunt for antigens.[14]
Neutrophils have a preference to engulf refined carbohydrates[15][16][17] (from ingested glucose, fructose, sucrose, honey and orange juice[15]) over bacteria.[15] In 1973 Sanchez et al. found that the neutrophil phagocytic capacity to engulf bacteria is affected when simple sugars are digested,[15] and that fasting strengthens the neutrophils' phagocytic capacity to engulf bacteria.[15] However, the digestion of normal starches has no effect. It was concluded that the function, and not the number, of phagocytes in engulfing bacteria was altered by the ingestion of sugars.[15] In 2007 researchers at the Whitehead Institute of Biomedical Research found that given a selection of sugars, neutrophils engulf some types of sugar preferentially.[16][17]
Development
Life span
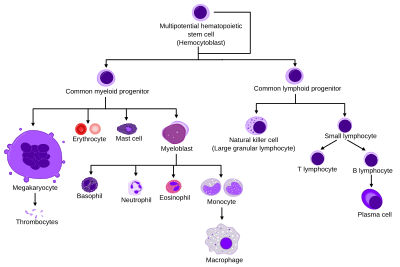
HSC=Hematopoietic stem cell, Progenitor=Progenitor cell, L-blast=lymphoblast, Lymphocyte, Mo-blast=Monoblast, Monocyte, Myeloblast, Pro-M=Promyelocyte, Myelocyte, Meta-M=Metamyelocyte, Neutrophil, Eosinophil, Basophil, Pro-E=Proerythroblast, Baso-E=Basophilic erythroblast, poly-e=Polychromatic erythroblast, Ortho-E=orthochromatic erythroblast, Erythrocyte, Promegakaryocyte, megakaryocyte, Platelet
The average lifespan of inactivated human neutrophils in the circulation has been reported by different approaches to be between 5 and 90 hours.[18] Upon activation, they marginate (position themselves adjacent to the blood vessel endothelium) and undergo selectin-dependent capture followed by integrin-dependent adhesion in most cases, after which they migrate into tissues, where they survive for 1–2 days.[19]
Neutrophils are much more numerous than the longer-lived monocyte/macrophage phagocytes. A pathogen (disease-causing microorganism or virus) is likely to first encounter a neutrophil. Some experts hypothesize that the short lifetime of neutrophils is an evolutionary adaptation. The short lifetime of neutrophils minimizes propagation of those pathogens that parasitize phagocytes because the more time such parasites spend outside a host cell, the more likely they will be destroyed by some component of the body's defenses. Also, because neutrophil antimicrobial products can also damage host tissues, their short life limits damage to the host during inflammation.[19]
Neutrophils will be removed after phagocytosis of pathogens by macrophages. PECAM-1 and phosphatidylserine on the cell surface are involved in this process.
Function
Chemotaxis
Neutrophils undergo a process called chemotaxis via amoeboid movement, which allows them to migrate toward sites of infection or inflammation. Cell surface receptors allow neutrophils to detect chemical gradients of molecules such as interleukin-8 (IL-8), interferon gamma (IFN-γ), C3a, C5a, and Leukotriene B4, which these cells use to direct the path of their migration.
Neutrophils have a variety of specific receptors, including ones for complement, cytokines like interleukins and IFN-γ, chemokines, lectins, and other proteins. They also express receptors to detect and adhere to endothelium and Fc receptors for opsonin.[20]
In leukocytes responding to a chemoattractant, the cellular polarity is regulated by activities of small Rho guanosine triphosphatases (Rho GTPases) and the phosphoinositide 3-kinases (PI3Ks). In neutrophils, lipid products of PI3Ks regulate activation of Rho GTPases and are required for cell motility. They accumulate asymmetrically to the plasma membrane at the leading edge of polarized cells. Spatially regulating Rho GTPases and organizing the leading edge of the cell, PI3Ks and their lipid products could play pivotal roles in establishing leukocyte polarity, as compass molecules that tell the cell where to crawl.
It has been shown in mice that in certain conditions neutrophils have a specific type of migration behaviour referred to as neutrophil swarming during which they migrate in a highly coordinated manner and accumulate and cluster to sites of inflammation.[21]
Anti-microbial function
Being highly motile, neutrophils quickly congregate at a focus of infection, attracted by cytokines expressed by activated endothelium, mast cells, and macrophages. Neutrophils express[22] and release cytokines, which in turn amplify inflammatory reactions by several other cell types.
In addition to recruiting and activating other cells of the immune system, neutrophils play a key role in the front-line defense against invading pathogens. Neutrophils have three methods for directly attacking micro-organisms: phagocytosis (ingestion), degranulation (release of soluble anti-microbials), and generation of neutrophil extracellular traps (NETs).[23]
Phagocytosis
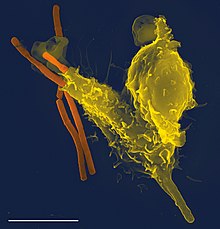
Scanning electron micrograph of a neutrophil (yellow) phagocytosing anthrax bacilli (orange). Scale bar, 5 μM
Neutrophils are phagocytes, capable of ingesting microorganisms or particles. For targets to be recognized, they must be coated in opsonins—a process known as antibody opsonization.[14] They can internalize and kill many microbes, each phagocytic event resulting in the formation of a phagosome into which reactive oxygen species and hydrolytic enzymes are secreted. The consumption of oxygen during the generation of reactive oxygen species has been termed the "respiratory burst", although unrelated to respiration or energy production.
The respiratory burst involves the activation of the enzyme NADPH oxidase, which produces large quantities of superoxide, a reactive oxygen species. Superoxide decays spontaneously or is broken down via enzymes known as superoxide dismutases (Cu/ZnSOD and MnSOD), to hydrogen peroxide, which is then converted to hypochlorous acid (HClO), by the green heme enzyme myeloperoxidase. It is thought that the bactericidal properties of HClO are enough to kill bacteria phagocytosed by the neutrophil, but this may instead be a step necessary for the activation of proteases.[24]
Degranulation
Neutrophils also release an assortment of proteins in three types of granules by a process called degranulation. The contents of these granules have antimicrobial properties, and help combat infection.
| Granule type | Protein |
Azurophilic granules (or "primary granules") | Myeloperoxidase, bactericidal/permeability-increasing protein (BPI), defensins, and the serine proteases neutrophil elastase and cathepsin G |
Specific granules (or "secondary granules") | Alkaline phosphatase, lysozyme, NADPH oxidase, collagenase, lactoferrin, histaminase,[25] and cathelicidin |
| Tertiary granules | Cathepsin, gelatinase and collagenase |
Neutrophil extracellular traps
In 2004, Brinkmann and colleagues described a striking observation that activation of neutrophils causes the release of web-like structures of DNA; this represents a third mechanism for killing bacteria.[26] These neutrophil extracellular traps (NETs) comprise a web of fibers composed of chromatin and serine proteases [27] that trap and kill extracellular microbes. It is suggested that NETs provide a high local concentration of antimicrobial components and bind, disarm, and kill microbes independent of phagocytic uptake. In addition to their possible antimicrobial properties, NETs may serve as a physical barrier that prevents further spread of pathogens. Trapping of bacteria may be a particularly important role for NETs in sepsis, where NETs are formed within blood vessels.[28] Recently, NETs have been shown to play a role in inflammatory diseases, as NETs could be detected in preeclampsia, a pregnancy-related inflammatory disorder in which neutrophils are known to be activated.[29] In addition, NETs are known to exhibit pro-thrombotic effects both in vitro[30] and in vivo.[31][32]
Clinical significance
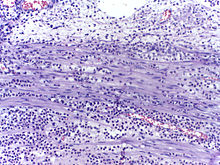
Micrograph showing several neutrophils during an acute inflammation.
Low neutrophil counts are termed neutropenia. This can be congenital (developed at or before birth) or it can develop later, as in the case of aplastic anemia or some kinds of leukemia. It can also be a side-effect of medication, most prominently chemotherapy. Neutropenia makes an individual highly susceptible to infections. It can also be the result of colonization by intracellular neutrophilic parasites.
In alpha 1-antitrypsin deficiency, the important neutrophil enzyme elastase is not adequately inhibited by alpha 1-antitrypsin, leading to excessive tissue damage in the presence of inflammation – the most prominent one being pulmonary emphysema. Negative effects of elastase has been also shown in cases when the neutrophils are excessively activated (in otherwise healthy individual) and release the enzyme in extracellular space. Unregulated activity of neutrophil elastase can lead to disruption of pulmonary barrier showing symptoms corresponding with acute lung injury.[33] The enzyme also influences activity of macrophages by cleaving their toll-like receptors (TLRs) and downregulating cytokine expression by inhibiting nuclear translocation of NF-κB.[34]
In Familial Mediterranean fever (FMF), a mutation in the pyrin (or marenostrin) gene, which is expressed mainly in neutrophil granulocytes, leads to a constitutively active acute-phase response and causes attacks of fever, arthralgia, peritonitis, and – eventually – amyloidosis.[35]
Decreases in neutrophil function have been linked to hyperglycemia. Dysfunction in the neutrophil biochemical pathway myeloperoxidase as well as reduced degranulation are associated with hyperglycemia.[36]
The Absolute neutrophil count (ANC) is also used in diagnosis and prognosis. ANC is the gold standard for determining severity of neutropenia, and thus neutropenic fever. Any ANC < 1500 cells / mm3 is considered neutropenia, but <500 cells / mm3 is considered severe.[37] There is also new research tying ANC to myocardial infarction as an aid in early diagnosis.[38][39]
Neutrophil antigens
There are five (HNA 1-5) sets of neutrophil antigens recognized.[40] The three HNA-1 antigens (a-c) are located on the low affinity Fc-γ receptor IIIb (FCGR3B :CD16b) The single known HNA-2a antigen is located on CD177. The HNA-3 antigen system has two antigens (3a and 3b) which are located on the seventh exon of the CLT2 gene (SLC44A2). The HNA-4 and HNA-5 antigen systems each have two known antigens (a and b) and are located in the β2 integrin. HNA-4 is located on the αM chain (CD11b) and HNA-5 is located on the αL integrin unit (CD11a).
Subpopulations

Activity of neutrophil-killer and neutrophil-cager in NBT-test.[41]
Two functionally unequal subpopulations of neutrophils were identified on the basis of different levels of their reactive oxygen metabolite generation, membrane permeability, activity of enzyme system, and ability to be inactivated. The cells of one subpopulation with high membrane permeability (neutrophil-killers) intensively generate reactive oxygen metabolites and are inactivated in consequence of interaction with the substrate, whereas cells of another subpopulation (neutrophil-cagers) produce reactive oxygen species less intensively, don't adhere to substrate and preserve their activity.[41][42][43][44][45]
Video
A rapidly moving neutrophil can be seen taking up several conidia over an imaging time of 2 hours with one frame every 30 seconds.
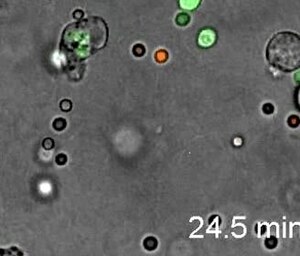 Play media
Play media
A neutrophil can be seen here selectively taking up several Candida yeasts (fluorescently labeled in green) despite several contacts with Aspergillus fumigatus conidia (unlabeled, white/clear) in a 3-D collagen matrix. Imaging time was 2 hours with one frame every 30 seconds.
[1] Neutrophils display highly directional amoeboid motility in infected footpad and phalanges. Intravital imaging was performed in the footpad path of LysM-eGFP mice 20 minutes after infection with Listeria monocytogenes.[46]
Additional images

Blood cell lineage

More complete lineages
References
^ Ermert D, Niemiec MJ, Röhm M, Glenthoj A, Borregard N, Urban CF (2013). "Candida albicans escapes mouse neutrophils". Journal of Leukocyte Biology. 94 (2): 223–36. doi:10.1189/jlb.0213063. PMID 23650619..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Witko-Sarsat, V; Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L (2000). "Neutrophils: molecules, functions and pathophysiological aspects". Lab Invest. 80 (5): 617–53. doi:10.1038/labinvest.3780067. PMID 10830774.CS1 maint: Multiple names: authors list (link)
^ Klebanoff, SJ; Clark, RA (1978). "The Neutrophil: Function and Clinical Disorders". Elsevier/North-Holland Amsterdam. ISBN 0-444-80020-4.
^ Nathan, C (Mar 2006). "Neutrophils and immunity: challenges and opportunities". Nature Reviews Immunology. 6 (March): 173–82. doi:10.1038/nri1785. ISSN 1474-1733. PMID 16498448.
^ Jacobs, L; Nawrot, Tim S; De Geus, Bas; Meeusen, Romain; Degraeuwe, Bart; Bernard, Alfred; Sughis, Muhammad; Nemery, Benoit; Panis, Luc (Oct 2010). "Subclinical responses in healthy cyclists briefly exposed to traffic-related air pollution". Environmental Health. 9 (64): 64. doi:10.1186/1476-069X-9-64. PMC 2984475. PMID 20973949.
^ Waugh, DJ; Wilson, C. (Nov 2008). "The interleukin-8 pathway in cancer". Clinical Cancer Research. 14 (21): 6735–41. doi:10.1158/1078-0432.CCR-07-4843. ISSN 1078-0432. PMID 18980965.
^ De Larco, JE; Wuertz, BR; Furcht, LT (Aug 2004). "The Potential Role of Neutrophils in Promoting the Metastatic Phenotype of Tumors Releasing Interleukin-8". Clinical Cancer Research. 10 (15): 4895–900. doi:10.1158/1078-0432.CCR-03-0760. ISSN 1078-0432. PMID 15297389.
^ Yoo, SK; Starnes, TW; Deng, Q; Huttenlocher, A (20 November 2011). "Lyn is a redox sensor that mediates leukocyte wound attraction in vivo". Nature. 480 (7375): 109–12. Bibcode:2011Natur.480..109Y. doi:10.1038/nature10632. PMC 3228893. PMID 22101434.
^ Barer, M.R. (2012). "The natural history of infection". Medical Microbiology. Elsevier. doi:10.1016/b978-0-7020-4089-4.00029-9. ISBN 978-0-7020-4089-4.
^ Cohen, Stephen; Burns, Richard C. (2002). Pathways of the Pulp (8th ed.). St. Louis: Mosby. p. 465.
^ Niemiec MJ, De Samber B, Garrevoet J, Vergucht E, Vekemans B, De Rycke R, Björn E, Sandblad L, Wellenreuther G, Falkenberg G, Cloetens P, Vincze L, Urban CF (2015). "Trace element landscape of resting and activated human neutrophils on sub-micrometer level". Metallomics. 7 (6): 996–1010. doi:10.1039/c4mt00346b. PMID 25832493.
^ abcd Zucker-Franklin, Dorothy; Greaves, M. F.; Grossi, C. E.; Marmont, A. M. (1988). "Neutrophils". Atlas of Blood Cells: Function and Pathology. 1 (2nd ed.). Philadelphia: Lea & Ferbiger. ISBN 0-8121-1094-3.
^ Reich, David; Nalls, Michael A.; et al. (30 January 2009). "Reduced Neutrophil Count in People of African Descent Is Due To a Regulatory Variant in the Duffy Antigen Receptor for Chemokines Gene". PLOS Genetics. 5 (1): e1000360. doi:10.1371/journal.pgen.1000360. ISSN 1553-7404. Retrieved 24 April 2017.
^ ab Edwards, Steven W. (1994). Biochemistry and physiology of the neutrophil. Cambridge University Press. p. 6. ISBN 0-521-41698-1.
^ abcdef Albert Sanchez; J. L. Reeser; H. S. Lau; P. Y. Yahiku; R. E. Willard; P. J. McMillan; S. Y. Cho; A. R. Magie; U. D. Register (1973). "Role of sugars in human neutrophilic phagocytosis". The American Society for Clinical Nutrition. Retrieved 2013-09-08.These data suggest that the function and not the number of phagocytes was altered by ingestion of sugars. This implicates glucose and other simple carbohydrates in the control of phagocytosis and shows that the effects last for at least 5 hr. On the other hand, a fast of 36 or 60 hr significantly increased (P < 0.001) the phagocytic index.
^ ab Rubin-Bejerano, I.; Abeijon, C.; Magnelli, P.; Grisafi, P.; Fink, G. R. (July 2007). "Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component". Cell Host Microbe. 2 (1): 55–67. doi:10.1016/j.chom.2007.06.002. PMC 2083279. PMID 18005717.
^ ab Kneller, Alyssa (2007). "White blood cells are picky about sugar". Whitehead Institute. Retrieved 2013-08-09.
^ Tak T, Tesselaar K, Pillay J, Borghans JA, Koenderman L (2013). "What's your age again? Determination of human neutrophil half-lives revisited". Journal of Leukocyte Biology. 94 (4): 595–601. doi:10.1189/jlb.1112571. PMID 23625199.
^ ab Wheater, Paul R.; Stevens, Alan (2002). Wheater's basic histopathology: a colour atlas and text (PDF)|format=requires|url=(help). Edinburgh: Churchill Livingstone. ISBN 0-443-07001-6.
^ Serhan, Charles N.; Ward, Peter A.; Gilroy, Derek W. (2010). Fundamentals of Inflammation. Cambridge University Press. pp. 53–54. ISBN 0-521-88729-1.
^ L?mmermann, Tim; Afonso, Philippe V.; Angermann, Bastian R.; Wang, Ji Ming; Kastenm?ller, Wolfgang; Parent, Carole A.; Germain, Ronald N. (26 May 2013). "Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo". Nature. 498 (7454): 371–375. Bibcode:2013Natur.498..371L. doi:10.1038/nature12175. PMC 3879961.
^ Ear, T; McDonald, PP (2008). "Cytokine generation, promoter activation, and oxidant-independent NF-kappaB activation in a transfectable human neutrophilic cellular model". BMC Immunol. 9: 14. doi:10.1186/1471-2172-9-14. PMC 2322942. PMID 18405381.
^ Hickey, MJ; Kubes, P (2009). "Intravascular immunity: the host–pathogen encounter in blood vessels". Nature Reviews Immunology. Nature Publishing Group. 9 (5): 364–75. doi:10.1038/nri2532. PMID 19390567.
^ Segal, AW (2005). "How neutrophils kill microbes". Annu Rev Immunol. 23 (5): 197–223. doi:10.1146/annurev.immunol.23.021704.115653. PMC 2092448. PMID 15771570.
^ Ringel, Eileen (1984). "Localization of histaminase to the specific granule of the human neutrophil". Immunology. 52 (4): 649–58. PMC 1454675. PMID 6430792.
^ Brinkmann, Volker; Ulrike Reichard, Christian Goosmann, Beatrix Fauler, Yvonne Uhlemann, David S. Weiss, Yvette Weinrauch, Arturo Zychlinsky (5 March 2004). "Neutrophil Extracellular Traps Kill Bacteria". Science. AAAS. 303 (5663): 1532–1535. Bibcode:2004Sci...303.1532B. doi:10.1126/science.1092385. ISSN 0036-8075. PMID 15001782. Retrieved 2007-04-09.CS1 maint: Multiple names: authors list (link)
^ Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A (2009). "Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans". PLOS Pathogens. 5 (10): e1000639. doi:10.1371/journal.ppat.1000639. PMC 2763347. PMID 19876394.
^ Clark SR, Ma AC, Tavener AS, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, DeVinney R, Doig CJ, Green FH, Kubes P (Apr 2007). "Platelet Toll-Like Receptor-4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Endotoxemic and Septic Blood" (PDF). Nature Medicine. Nature Publishing Group. 13 (4): 463–9. doi:10.1038/nm1565. ISSN 1078-8956. PMID 17384648.
^ Gupta, AK; Hasler, P; Holzgreve, W; Hahn, S (Jun 2007). "Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia?". Semin Immunopathol. 29 (2): 163–7. doi:10.1007/s00281-007-0073-4. ISSN 1863-2297. PMID 17621701.
^ Fuchs, TA; Brill, A, Duerschmied, D, Schatzberg, D, Monestier, M, Myers DD Jr, Wrobleski, SK, Wakefield, TW, Hartwig, JH, Wagner, DD (Sep 7, 2010). "Extracellular DNA traps promote thrombosis". Proceedings of the National Academy of Sciences of the United States of America. 107 (36): 15880–5. Bibcode:2010PNAS..10715880F. doi:10.1073/pnas.1005743107. PMC 2936604. PMID 20798043.CS1 maint: Multiple names: authors list (link)
^ Brill, A; Fuchs, TA, Savchenko, A, Thomas, GM, Martinod, K, De Meyer, SF, Bhandari, AA, Wagner, DD (Nov 1, 2011). "Neutrophil Extracellular Traps Promote Deep Vein Thrombosis in Mice". Journal of Thrombosis and Haemostasis. 10 (1): 136–44. doi:10.1111/j.1538-7836.2011.04544.x. PMC 3319651. PMID 22044575.CS1 maint: Multiple names: authors list (link)
^ Borissoff, JI; ten Cate, H (September 2011). "From neutrophil extracellular traps release to thrombosis: an overshooting host-defense mechanism?". Journal of Thrombosis and Haemostasis. 9 (9): 1791–4. doi:10.1111/j.1538-7836.2011.04425.x. PMID 21718435.
^ Kawabata, Kazuhito; Hagio, Tetsuya; Matsuoka, Shozo (2002-09-06). "The role of neutrophil elastase in acute lung injury". European Journal of Pharmacology. 451 (1): 1–10. ISSN 0014-2999. PMID 12223222.
^ Domon, Hisanori; Nagai, Kosuke; Maekawa, Tomoki; Oda, Masataka; Yonezawa, Daisuke; Takeda, Wataru; Hiyoshi, Takumi; Tamura, Hikaru; Yamaguchi, Masaya (2018). "Neutrophil Elastase Subverts the Immune Response by Cleaving Toll-Like Receptors and Cytokines in Pneumococcal Pneumonia". Frontiers in Immunology. 9. doi:10.3389/fimmu.2018.00732. ISSN 1664-3224.
^ Ozen, S (Jul 2004). "Familial mediterranean fever: revisiting an ancient disease". European Journal of Pediatrics. 162 (7–8): 449–54. doi:10.1007/s00431-003-1223-x. ISSN 0340-6199. PMID 12751000.
^ Xiu, Fangming; Stanojcic, Mile; Diao, Li; Jeschke, Marc G. (8 May 2014). "Stress Hyperglycemia, Insulin Treatment, and Innate Immune Cells". International Journal of Endocrinology. 2014: 9. doi:10.1155/2014/486403. Retrieved 19 January 2016.
^ Al-Gwaiz, LA; Babay, HH (2007;16(5):344-7.). "The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections". Med Princ Pract. 16 (5): 344–7. doi:10.1159/000104806. PMID 17709921. Check date values in:|date=(help)
^ Khan, HA; et al. (2012). "Blood cell counts and their correlation with creatine kinase and C-reactive protein in patients with acute myocardial infarction". Int J Clin Exp Med. 5 (1): 50–5. PMC 3272686. PMID 22328948.
^ Basili, S; et al. (2004). "Absolute neutrophil counts and fibrinogen levels as an aid in the early diagnosis of acute myocardial infarction". Acta Cardiol. 59 (2): 135–40. doi:10.2143/ac.59.2.2005167. PMID 15139653.
^ Chu HT, Lin H, Tsao TT, Chang CF, Hsiao WW, Yeh TJ, Chang CM, Liu YW, Wang TY, Yang KC, Chen TJ, Chen JC, Chen KC, Kao CY (2013). "Genotyping of human neutrophil antigens (HNA) from whole genome sequencing data". BMC Med. Genom. 6 (1): 31. doi:10.1186/1755-8794-6-31. PMC 3849977. PMID 24028078.
^ ab Ignatov, Dmitry Yu. (2012). Functional heterogeneity of human neutrophils and their role in peripheral blood leukocyte quantity regulation (PhD). Donetsk National Medical University. doi:10.13140/RG.2.2.35542.34884.
^ Gerasimov I.G., Ignatov D.Yu. (2001). "Functional heterogenicity of human blood neutrophils: generation of oxygen active species". Tsitologiia. 43 (5): 432–436. PMID 11517658.
^ Gerasimov I.G., Ignatov D.Yu. (2004). "Neutrophil activation in vitro". Tsitologiia. 46 (2): 155–158. PMID 15174354.
^ Gerasimov I.G., Ignatov D.Yu. (2005). "Nitroblue tetrazolium reduction by human blood neutrophils. I. The influence of pH". Tsitologiia. 47 (6): 549–553. PMID 16708848.
^ Gerasimov I.G., Ignatov D.Yu. (2005). "Nitroblue tetrazolium reduction by human blood neutrophils. II. The influence of sodium and potassium ions". Tsitologiia. 47 (6): 554–558. PMID 16708849.
^ Graham D.B., Zinselmeyer B.H., Mascarenhas F., Delgado R., Miller M.J., Swat W.; Zinselmeyer; Mascarenhas; Delgado; Miller; Swat (2009). Unutmaz, Derya, ed. "ITAM signaling by Vav family Rho guanine nucleotide exchange factors regulates interstitial transit rates of neutrophils in vivo". PLoS ONE. 4 (2): e4652. Bibcode:2009PLoSO...4.4652G. doi:10.1371/journal.pone.0004652. PMC 2645696. PMID 19247495.CS1 maint: Multiple names: authors list (link)
External links
- Neutropenia Information
- Absolute Neutrophil Count Calculator
- Neutrophil Trace Element Content and Distribution


