Acetoacetyl-CoA
 | |
| Identifiers | |
|---|---|
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChemSpider |
|
ECHA InfoCard | 100.014.378 |
IUPHAR/BPS |
|
MeSH | acetoacetyl+CoA |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
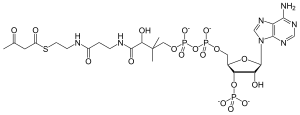
Chemical formula | C25H40N7O18P3S |
Molar mass | 851.609 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Acetoacetyl CoA is the precursor of HMG-CoA in the mevalonate pathway, which is essential for cholesterol biosynthesis. It also takes a similar role in the ketone bodies synthesis (ketogenesis) pathway of the liver. In the ketone bodies digestion pathway (in the tissue), it is no longer associated with having HMG-CoA as a product or as a reactant.
It is created from acetyl-CoA, a thioester, which reacts with the enolate of a second molecule of acetyl-CoA in a Claisen condensation reaction,[1] and it is acted upon by HMG-CoA synthase to form HMG-CoA. During the metabolism of leucine, this last reaction is reversed.

References
^ Yurkanis, Bruice, Paula (2017). Organic chemistry. Pearson. ISBN 9780134042282. OCLC 974910578..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
See also
- Mevalonate pathway
- Acetoacetic acid
- Beta-hydroxybutyryl-CoA dehydrogenase
This biochemistry article is a stub. You can help Wikipedia by expanding it. |